-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2015; 5(1): 1-6
doi:10.5923/j.nn.20150501.01
Synthesis of Zinc Oxide Nanoparticles via Sol – Gel Route and Their Characterization
Riyadh M. Alwan, Quraish A. Kadhim, Kassim M. Sahan, Rawaa A. Ali, Roaa J. Mahdi, Noor A. Kassim, Alwan N. Jassim
National Center for Packing and Packaging, Corporation of research and industrial development, Iraqi Ministry of industry and minerals
Correspondence to: Alwan N. Jassim, National Center for Packing and Packaging, Corporation of research and industrial development, Iraqi Ministry of industry and minerals.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
In this work, zinc oxide nanoparticles were readily synthesized through sol-gel method using zinc acetate as a precursor. The crystalline structure, morphology of synthesized ZnO nanoparticles were observed using powder X-ray diffraction (XRD), FTIR analysis, scanning electron microscopy (SEM) and their optical properties characterized using UV -visible spectroscopy.XRD results revealed that the prepared ZnO sample is highly crystalline, having wurtzite crystalstructure. FT-IR spectra peak at 417.52 cm-1 indicated characteristic absorption bands OF ZnO nanoparticles. UV-Vis absorption spectrum showed a typical spectrum for ZnO nanoparticles. The SEM image shows that ZnO nanoparticles prepared in this study are spherical in shape with smooth surface.
Keywords: ZnO nanoparticles, Sol-gel, SEM & XRD
Cite this paper: Riyadh M. Alwan, Quraish A. Kadhim, Kassim M. Sahan, Rawaa A. Ali, Roaa J. Mahdi, Noor A. Kassim, Alwan N. Jassim, Synthesis of Zinc Oxide Nanoparticles via Sol – Gel Route and Their Characterization, Nanoscience and Nanotechnology, Vol. 5 No. 1, 2015, pp. 1-6. doi: 10.5923/j.nn.20150501.01.
Article Outline
1. Introduction
- Due to novel properties like high refractive index, binding energy, high thermal conductivity, antibacterial and UV- protection of ZnO, it could be used in many materials and products. The products include medicine, cosmetics, rubber, solar cells and foods [1]. Zinc oxide has high biocompatibility and fast electric transfer kinetics, such phenomena encourage the use of this material as a biomimic membrane to immobilize and modify the biomolecules [2].In many literatures, it can be learned that nano ZnO offers better performance compared to that of bulk size [3]. Zinc is a necessary element to our health and ZnO nano particles also have good biocompatibility to human cells [4].Recently ZnO is listed as generally documented as safe material by FDA (food and drug administration, (US A) [5, 6].Ceramic powders like MgO, CaO, TiO2 and ZnO were found to inhibit strongly bacterial growth [7]. Many methods have been used to prepare ZnO nanoparticles like sol-gel method [8-15], thermal decomposition, chemical vapor decomposition (CVD) and alloy evaporation-deposition [16-22].A simple, fast wet chemical route based on cyclohexyl amine for synthesizing zinc oxide nanoparticles in aqueous and ethanolic media was established by Abdul-Aziz (2013). Particles of polyhedra morphology were obtained for ZnO prepared in ethanol, while spherical and some chunky particles were obtained for zinc oxide prepared in water. [23].Bari (2009) [24], has observed that when NH4OH is used as the solvent for zinc acetate to synthesis nano ZnO particles, the particles are spherical, while the particles are wire like when sodium hydroxide is used as solvent. Also, the results of Zaborski (2010) [25] revealed the morphology of ZnO which was prepared in the presence of the ionic liquids is spherical while it changes to plate-like without ionic liquids.It demonstrated that ZnO with different morphologies such as flowers and rods can be controllable obtained by simply varying the basicity in the solution. [26]Eric (1998), found that ZnO nanoparticles continue to grow after synthesis, even when stored at 0C°. The ability to obtain various particle sizes is based on this phenomenon. Also, it was found that the solution composition and temperature have a marked influence on the rate of the particle growth. [27]In brief, the solvents, temperature and media of experiment affect the particle size and particle morphology of synthesized ZnO nanoparticles. The aim of this research was to find a simple route to prepare nano ZnO particles viaSol- Gel method and characterize the final product using several techniques.
2. Experimental Section
- All the chemical reagents in this experiment were obtained from commercial sources as guaranteed – grade, and were used as received without further treatment.In our experiment, the sol - gel method was used for preparation of zinc oxide nanoparticles (ZnO-NPs). In a typical procedure 12.6g of zinc acetate dihydrate was added to 400 ml of double distilled water with continuous stirring to dissolve zinc acetate completely. Then the solution was heated to 50℃ and 600 ml of absolute alcohol was added slowly with stirring. After this, 6ml of H2O2 (% 47) was added dropwise to the vessel and mixed it using a magnetic stirrer to get an almost clear solution. This solution was incubated for 24 hours and the solution was dried at 80℃ for several hours to obtainwhite nano zinc oxide.Nano zinc oxide was washed several times with double distilled water to remove the byproducts. After washing, the ZnO nanoparticles were dried at 80℃ in hot air oven. Complete conversion of zinc oxide will occur during the drying process.
3. Physical and Physico – chemical Characterization
- Morphology of the sample was investigated using scanning electron microscope (SEM). Specimens were prepared by dispersing ZnO nanoparticles in absolute ethanol under ultrasonic stirring, dropping some of the solution onto a glass slide, and evaporating the solvent naturally in air. Then these specimens were sputter coated with a thin gold layer of about 3 nm thick in vacuum. The crystallinity was determined by XRD powder diffraction. Analysis was performed by using an XRD SHIMADZU 6000 diffractometer equipped with a Cukα (K=1.54 A°) source, maintaining applied voltage of 40 kV and current at 30 mA. About 0.3 g of dried ZnO particles were deposited as a randomly oriented powder into a plexiglass sample container, and the XRD patterns were recorded between 5°and 50° angles, with speed of 5.0 deg /min.The crystalline domain diameter (D) was obtained from XRD peaks using the following Scherrer's equation [28]: D=K * λ /β*cosθWhere λ is the wavelength of the incident X-ray beam; Ɵ the Bragg's diffraction angle; β the width of the X-ray pattern line at half peak – height in radian and the dimensionless shape factor (K) has a typical value of 0.89, but varies with the actual shape of the crystalline [14].Inductive coupled plasma (ICP-OES spectrometer 725 series-Agilent Technologies) was used to determine the concentration of Zn.The reaction yield was calculated by measuring the concentration of Zn in the solution before and after the completion of the reaction.The UV- Vis absorption of the samples was recorded using an automated spectrometer (Spectro UV-VIS Double beam UVD-3500) in the wavelength range 190nm -900nm.ZnO powder was analyzed using FTIR (model Jasco-4200). A disk of 1:3 ratio of KBr was prepared with a mixture of dried ZnO and then examined under IR Spectrometer. Infrared spectra were recorded in the region of 400to 4000 cm-1.
4. Results and Discussion
- X-ray diffraction analysis The phase purity and composition of the particles obtained by a sol - gel process examined by XRD. Figure (1) shows a typical XRD pattern of ZnO nanoparticles, prepared in this work.
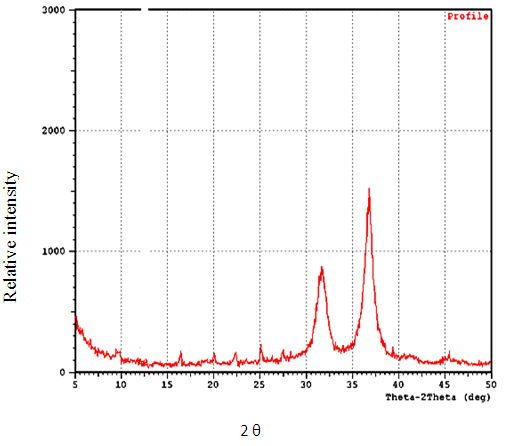 | Figure 1. Shows Typical XRD pattern of ZnO nanonanoparticls |
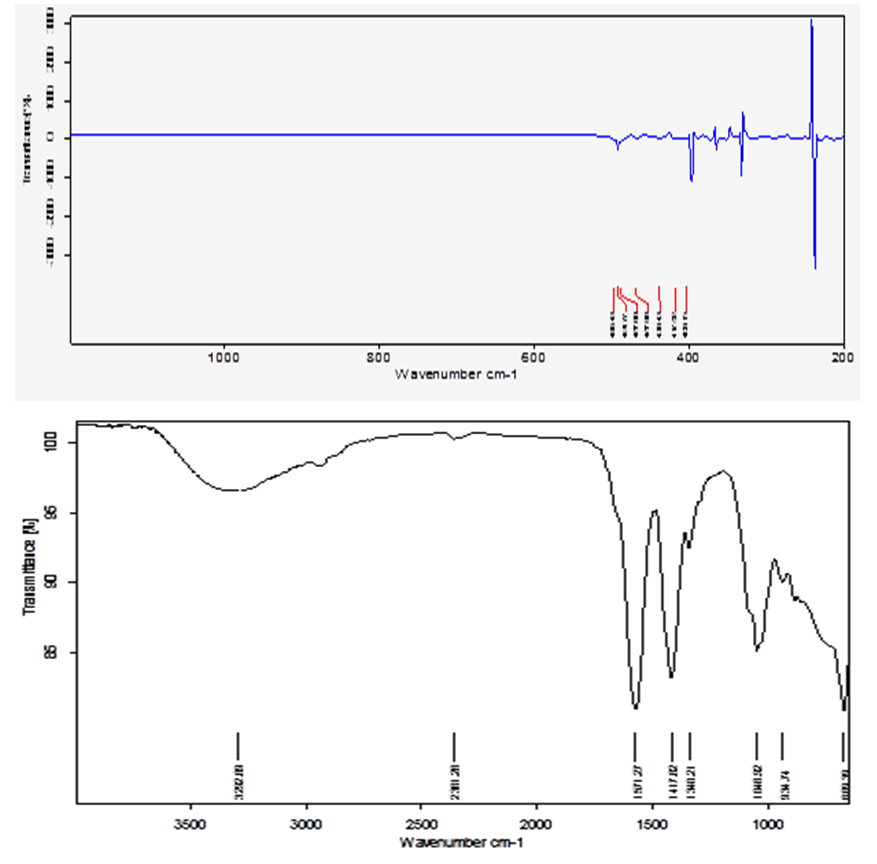 | Figure 2. FTIR Transmition spectra of pure ZnO nanoparticls |
 | Figure 3. Shows the SEM image of ZnO nanoparticles |
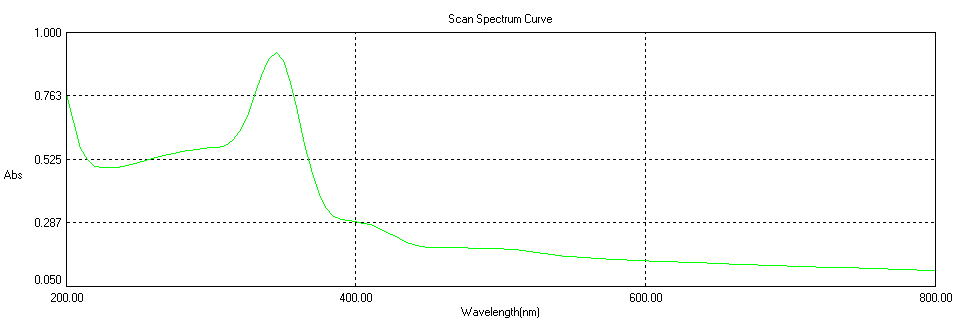 | Figure 4. UV-Vis optical absorption spectrum of ZnO nano particles |
 | Figure 5. DRS plot for finding the band gap of ZnO |
5. Conclusions
- ZnO nanoparticles have been successfully synthesized by simple Sol-Gel method. The prepared ZnO nanoparticles were spherical in shape and were characterized using XRD, UV-Vis absorption, FT-IR and SEM techniques. The average particle size was found to be 58.3 nm using Scherrer's equation and 100-200 nm obtained from SEM measurement for ZnO nano particles dried at 80℃.ZnO nanoparticales offer tremendous potential in future applications of electronic and magneto–electric devices. Also, maybe, applied for photocatalysis, gas sensing, biomedical device and sun sucreen applications. The method has a high yield and can be used for large scale synthesis of ZnO nano particles [51].
ACKNOWLEDGEMENTS
- This work was gratefull supported by the Iraqi ministry of industry and minerals – Directory of planning in the frame work of grant No. 197 in October 2012.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML