-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2014; 4(1): 16-21
doi:10.5923/j.nn.20140401.03
Size-Dependent Photoluminescence Quantum Yield of Cdse/Zns Nanoparticles: Numerical Simulation and Experimental Evidence
Natalia Strekal
Physics and Technics Department, Yanka Kupala State University, Grodno, 230023, Belarus
Correspondence to: Natalia Strekal, Physics and Technics Department, Yanka Kupala State University, Grodno, 230023, Belarus.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The fundamental problem concerning the propagation of quantum size effect on parameters which determine electron transition probabilities in CdSe/ZnS nanoparticles has been solved. The firstly obtained size-dependent relative fluorescent quantum yield in CdSe/ZnS nanoparticles is interpreted as indication of defect-free crystal structure in nanoparticles and is suggested as calibrating curve for quality control during the nanoparticle synthesis.
Keywords: CdSe/ZnS nanoparticles, Fluorescence quantum yield, Decay time
Cite this paper: Natalia Strekal, Size-Dependent Photoluminescence Quantum Yield of Cdse/Zns Nanoparticles: Numerical Simulation and Experimental Evidence, Nanoscience and Nanotechnology, Vol. 4 No. 1, 2014, pp. 16-21. doi: 10.5923/j.nn.20140401.03.
Article Outline
1. Introduction
- Semiconductor nanoparticles (NP), or quantum dots (QD) represent a unique class of semiconductor particles with sizes ranging between 2-10 nm in diameter. Within this size range, materials reveal unique optical and electronic properties resulting from quantum size effects. At present, greatest attention within semiconductor group АIIBVI [2; 3; 4] is paid to composite nanocrystals CdSe/ZnS of the core-shell type. Well-established and time-spaced synthesis phases [2] include nucleation and growth processes, and allow to obtain CdSe particles properly selected by size. Owing to their well-known advantages over organic fluorophores, CdSe / ZnS QDs are regarded as promising candidates to serve as a basis for development of new types of light-emitting devices [5; 6], biological object markers [7-10], and multi-colour laser devices [11].The purpose of this study is theoretical and experimental analysis of quantum characteristics of fluorescent composite CdSe/ZnS NPs with cadmium selenide core and zinc sulphide shell, depending on CdSe core diameter. The notion of quantum characteristics implies a set of energy eigenvalues and a set of eigenfunctions of nonequilibrium charge carrier. The former of these sets is characterized by a well-known dependence on the nanoparticle sizes, which is interpreted within the so-called quantum size effect. It is natural to suppose that the latter, the set of eigenfunctions, should similarly be inherently dependent on nanoparticle sizes, if only because both sets are internally related and are different “sides” of the same solution. However, while dependence between the range of charge carriers’ eigenenergy values in nanoparticles and the nanoparticle sizes is widely used as a calibration curve in estimation of the said sizes, the dependence between the eigenfunctions range and nanoparticle sizes is still not even established with sufficient reliability. To establish this dependence based on calculations and measurements, as well as to apply the obtained dependence to solution of practical problems are the purposes of this study.
2. Calculations
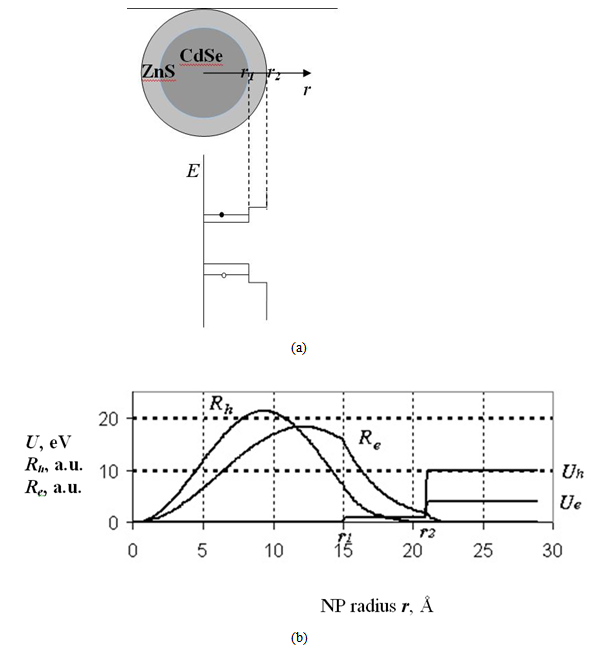
- The quantum confinement effect of non-equilibrium charge carriers in nano-sized semiconductor particle causes changes in the whole range of its physical properties. This effect is sufficiently visible as nanostructure dimensions become smaller than or comparable to a certain parameter inherent to a given material, like, for example, Bohr exciton radius or electron wave length. There are also necessary conditions under which the probability of observing the quantum confinement effect increases as charge carrier scattering phenomenon appears. Such necessary conditions are the presence of a perfect crystalline structure, density and charge carriers’ free path length.We have developed a computer program to realize a quantum “particle in the well” model [12] with step potential. A modified version of the program uses effective mass and barrier energy values of charge carriers in cadmium selenide and zinc sulphide. Dependences between major quantum characteristics of composite CdSe/ZnS nanoparticles and their sizes have been built for varying CdSe core diameter and ZnS shell thickness. It should be noted that earlier the authors of this study [13] also used the simplest model of charge carrier localization in the potential well for interpretation of findings of nanoparticles’ physical and chemical property studies, including optical electron spectroscopy and X-ray photoelectron spectroscopy, X-ray structure studies within small and large angles of X-ray incidence and dispersion, transmission electron microscopy. This allowed us to explain why CdSe/ZnS nanoparticles have a higher quantum yield as compared to CdSe/CdS nanoparticles. Besides, we were able to demonstrate why ZnS shell is more advantageous than CdS shell. These facts are not trivial from the point of view of chemical stoichiometry and formation of dislocations at the core-shell border. However, zinc sulphide passivation provides a potential barrier increase for charge carriers at the border and facilitates an increased quantum yield of photoluminescence (PL) due to greater charge localization in the core. Besides, the choice of core thickness, optimal with regard to quantum-optical characteristics of nanoparticles, too, has been demonstrated by the authors of this study on the basis of a simple quantum-mechanical “particle in the well” model under the effective-mass approximation of non-interacting charge carriers.Analysis of the charge carrier’s eigenfunctions is paid less attention to, though studies of size-dependence of spectral characteristics, such as transition oscillator strength and recombination probabilities of photo-excited charge carriers, require information on spatial overlap of the charge carriers’ eigenfunctions. Let us consider the statement of the problem and the solutions obtained. The Schrodinger equation in the case of rectangular two-step potential well is presented as (Fig. 1):
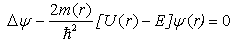 | (1) |
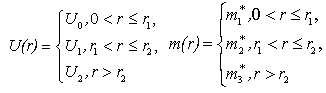 | (2) |
 ) and for the hole (with potential
) and for the hole (with potential 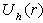 ) independently.
) independently.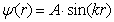 or
or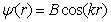 .Outside the well, physically acceptable solutions have the form of:
.Outside the well, physically acceptable solutions have the form of: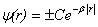 ,where
,where 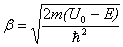 . The final solution with a view to the radial density of probability is presented as
. The final solution with a view to the radial density of probability is presented as 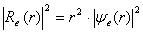 for the electron and as
for the electron and as 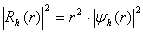 for the hole. Proceeding from requirements of continuity and smoothness, matching should be provided at the potential borders, i.e., parameters
for the hole. Proceeding from requirements of continuity and smoothness, matching should be provided at the potential borders, i.e., parameters  should ensure that at the border area both the wave function and its derivative have the same values on both sides of the border. Since these conditions are only realized for certain
should ensure that at the border area both the wave function and its derivative have the same values on both sides of the border. Since these conditions are only realized for certain 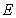 values, a particle localized in a potential well has a discrete energy spectrum.In our calculations, the Euler-Cromer method was supplemented with the requirement of constancy of probability current at the core-shell and shell-matrix borders.The following parameters have been taken as initial for calculations were:in CdSe core -
values, a particle localized in a potential well has a discrete energy spectrum.In our calculations, the Euler-Cromer method was supplemented with the requirement of constancy of probability current at the core-shell and shell-matrix borders.The following parameters have been taken as initial for calculations were:in CdSe core - 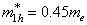 ,
, 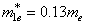 ,
, 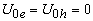 eV;at the core-shell border -
eV;at the core-shell border - 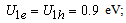 in the organic matrix -
in the organic matrix - 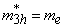 ,
, 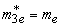 ;at the shell-matrix border -
;at the shell-matrix border - 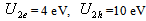 ,where
,where 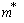 is the effective mass, indices e and h indicate the parameter’s correspondence to the electron or the hole.The shape of the well for the electron and the hole localized in a nanoparticle with CdSe core radius 15 Å and ZnS shell thickness 6 Å is given in Fig. 1. Fig. 1 also demonstrates radial probability densities Re and Rh for the electron and the hole. The hole, being a heavier particle, is seen to be localized closer to the core center. A kink is clearly visible on the electron’s radial probability density graph in the core-shell border area. It is less pronounced for the hole and results from an abrupt change of the charge carriers’ effective mass at this point.Quantum confinement effects on the photoexcited charge carrier energy and electron transition oscillator strength have been studied theoretically by L.E. Bruce in [14]. Within very small crystallites (∼50 Å in diameter), a solution was obtained for Schrodinger equation with regard to Wannier excitons. In the process, kinetic energy operator was recorded using effective masses data for the electron and the hole, while potential energy operator was recorded using the expression provided by high-frequency dielectric electron solvation of atom cores. Oscillator strength of the electron transition assumes the following form:
is the effective mass, indices e and h indicate the parameter’s correspondence to the electron or the hole.The shape of the well for the electron and the hole localized in a nanoparticle with CdSe core radius 15 Å and ZnS shell thickness 6 Å is given in Fig. 1. Fig. 1 also demonstrates radial probability densities Re and Rh for the electron and the hole. The hole, being a heavier particle, is seen to be localized closer to the core center. A kink is clearly visible on the electron’s radial probability density graph in the core-shell border area. It is less pronounced for the hole and results from an abrupt change of the charge carriers’ effective mass at this point.Quantum confinement effects on the photoexcited charge carrier energy and electron transition oscillator strength have been studied theoretically by L.E. Bruce in [14]. Within very small crystallites (∼50 Å in diameter), a solution was obtained for Schrodinger equation with regard to Wannier excitons. In the process, kinetic energy operator was recorded using effective masses data for the electron and the hole, while potential energy operator was recorded using the expression provided by high-frequency dielectric electron solvation of atom cores. Oscillator strength of the electron transition assumes the following form: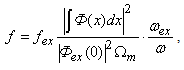 | (3) |
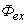 is the wave function 1s of the hydrogen atom state,
is the wave function 1s of the hydrogen atom state, 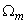 is the volume of a single two-atom group CdS in crystallite, fex= 0.00256 is the oscillator strength value of bulk cadmium sulphide, and
is the volume of a single two-atom group CdS in crystallite, fex= 0.00256 is the oscillator strength value of bulk cadmium sulphide, and 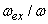 is radiation frequencies ratio of bulk cadmium sulphide and small crystallite CdS. The obtained expression demonstrates that in small crystallites oscillator strength of electron transition only slightly depends on radiation frequency
is radiation frequencies ratio of bulk cadmium sulphide and small crystallite CdS. The obtained expression demonstrates that in small crystallites oscillator strength of electron transition only slightly depends on radiation frequency 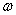 .Expression (3) for transition oscillator strength was presented by L.E. Brus for small CdS crystallytes. However, it is of a practically universal nature as it demonstrates the nature of dependence between the transition oscillator strength and the wave function overlap integral of photoexcited charge carriers, and thus contains information regarding the effects of quantum restraints on optical transition parameters. Oscillator strength is related to optical density, according to [15], as f = 4,32 10-9 A, optical density A being expressed as molar extinction as per the well-known Bouguer-Lambert-Beer absorption law.Figure 2 demonstrates dependencies between overlap integrals
.Expression (3) for transition oscillator strength was presented by L.E. Brus for small CdS crystallytes. However, it is of a practically universal nature as it demonstrates the nature of dependence between the transition oscillator strength and the wave function overlap integral of photoexcited charge carriers, and thus contains information regarding the effects of quantum restraints on optical transition parameters. Oscillator strength is related to optical density, according to [15], as f = 4,32 10-9 A, optical density A being expressed as molar extinction as per the well-known Bouguer-Lambert-Beer absorption law.Figure 2 demonstrates dependencies between overlap integrals 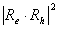 and CdSe/ZnS nanoparticle diameters, calculated for the “particle in the well” model.
and CdSe/ZnS nanoparticle diameters, calculated for the “particle in the well” model.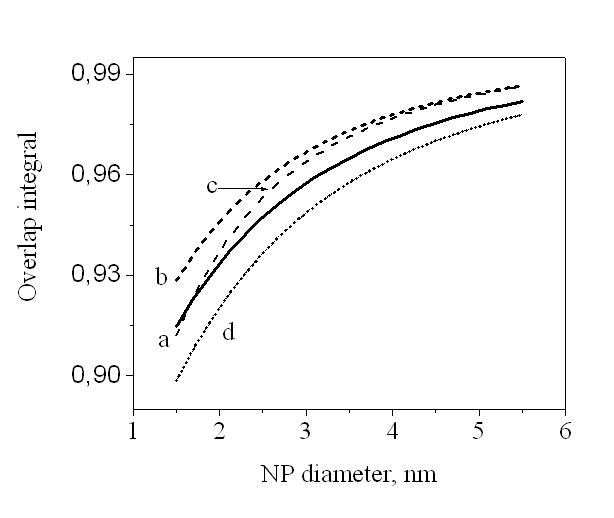 | Figure 2. Overlap integrals for eigenfunctioms of electron and hole in spherically symmetric nanoparticle CdSe/ZnS: (a) rectangular step potential; (b-d deformed on the shell surface |
3. Experimental Data
- Let us now address experimental data. Fig. 3 demonstrates spectra of hydrophobic CdSe nanoparticles obtained from the manufacturing company Evidot as toluene dispersions, with CdSe core sizes ranging between 2.1 and 4.0 nm. The values of nanoparticle sizes were obtained from Evidot’s specification. Optical densities (Fig. 3, a) have the same values in all samples, with excitation wave length being 400 nm. PL spectra obtained under excitation at 400 nm are demonstrated in Fig. 3, b. The edge of the nanoparticle absorption band (Fig. 3, a) and the PL position band, depending on their sizes, tend to shift towards the shorter wavelength area as a manifestation of the quantum-size effect.
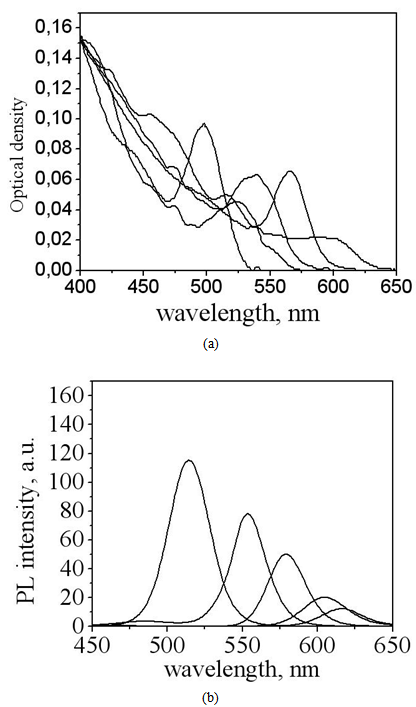 | Figure 3. Electronic spectra of CdSe/ZnS NP, dispersed in toluene: (a) optical density spectra; (b) PL spectra; 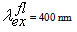 |
 , on the one hand, and diameters d of toluene-dispersed CdSe/ZnS nanoparticles, on the other hand. PL mean decay time
, on the one hand, and diameters d of toluene-dispersed CdSe/ZnS nanoparticles, on the other hand. PL mean decay time  monotonously decreases as nanoparticle diameters d increase, similar to decrease in PL relative intensity (Fig. 3, b) as nanoparticle sizes increase.
monotonously decreases as nanoparticle diameters d increase, similar to decrease in PL relative intensity (Fig. 3, b) as nanoparticle sizes increase.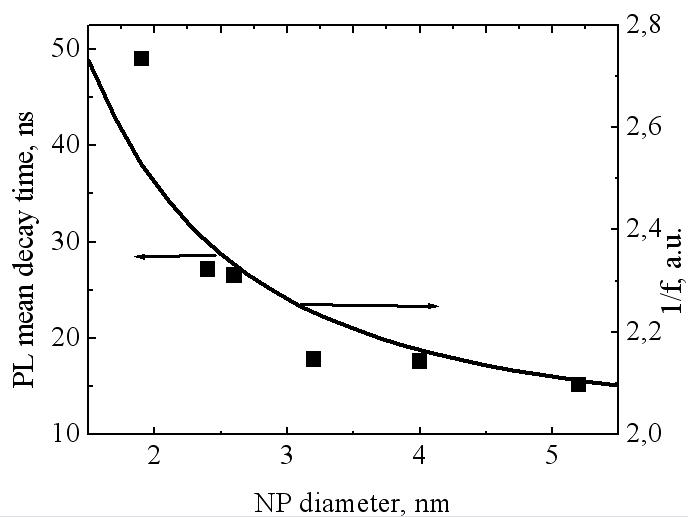 | Figure 4. Dependence of quantum transition probabilities (1/f) and PL mean decay time 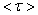 (small squares) for CdSe/ZnS NP dispersed in toluene on there diameter d (small squares) for CdSe/ZnS NP dispersed in toluene on there diameter d |
4. Results and Discussion
- Available published evidence on measurements of absorption cross-section, oscillator strength, and extinction coefficient with regard to nanoparticles is highly contradictory. Thus, for CdS nanoparticles, the authors of [16] presented dependence between the measured oscillator strength values of 1Se − 1S3/2h transition and radius r of nanoparticles within 5 to 1 nm range, including strong quantum confinement range.It turned out that the sizes smaller than Bohr exciton radius aB (about 2.7 nm in the case of CdS [1]) demonstrate an increase in transition oscillator strength as the radius decreases. Experimental data were well approximated by the function
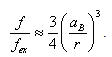 | (4) |
5. Conclusions
- A correlation has been established for the first time between theoretical and experimental data regarding dependence between the electron transition oscillator strength and the relative PL quantum yield, on the one hand, and CdSe/ZnS nanoparticle sizes, on the other hand. The established quantum-size dependence of the electron transition oscillator strength may be interpreted as an indicator of the preferential radiative electron and hole recombination channel between the lowest 1Se − 1S3/2h levels in hydrophobic CdSe/ZnS nanoparticles. This, in its turn, may be characteristic for nanoparticles with a perfect crystal phase.
ACKNOWLEDGEMENTS
- The study has been performed with financial support from Belarusian National Foundation for Fundamental Research (grants F05К-140, F07К-094, F10Р-232 и F11ОB-121).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML