-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2013; 3(5): 123-129
doi:10.5923/j.nn.20130305.03
Influence of Deposition Temperature and Pressure on Structural and Electrical Properties of Boron Doped Poly-Si13Ge87 Films Grown by Chemical Vapour Deposition
T. B. Asafa
KACST-Intel Consortium Center of Excellence in Nano-manufacturing Applications, Saudi Arabia, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Oyo State, Nigeria
Correspondence to: T. B. Asafa, KACST-Intel Consortium Center of Excellence in Nano-manufacturing Applications, Saudi Arabia, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Oyo State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The structuraland electrical properties of heavily doped, ~100nm-thick poly-SiGefilmsdeposited by a low pressure chemical vapour deposition system were investigated. For the films with a germanium fraction of ~87% and a boron concentration of ~3.47x1021 atoms/cm3, an increase inthe deposition temperature from 390 to 455oC raises the deposition rate from 18 to 28 nm/min and Hall mobility from ~2.2 to ~6.4 cm2V-1s-1. Higher deposition temperature lowers the film resistivity from 1.6 to 0.9 mΩcm, significantly enhances the film crystallinity andimproves carrier concentration from 1.65x1021 to 1.05x1021 atoms/cm3. By using Arrhenius plot, the thermal activation energy for poly-SiGe (~160 meV) is found to be lower than that of epitaxial SiGe (~200 meV). Increasing chamber pressure from 50 to 80 Torr does not significantly influence the crystallinity, grain size and resistivity of the films. However, it slightly enhances the Hall mobility and deposition rate. In practice, a structural layer having low resistivity and high mobility is desired for nanoelectromechanical layers such as nanoswitches and nanoresonators which require low pull-in and pull-out voltages for efficient performance.
Keywords: Poly-SiGe, Deposition temperature, Hall mobility, Resistivity
Cite this paper: T. B. Asafa, Influence of Deposition Temperature and Pressure on Structural and Electrical Properties of Boron Doped Poly-Si13Ge87 Films Grown by Chemical Vapour Deposition, Nanoscience and Nanotechnology, Vol. 3 No. 5, 2013, pp. 123-129. doi: 10.5923/j.nn.20130305.03.
Article Outline
1. Introduction
- Polycrystalline silicon germanium (poly-SiGe) films are now being used to fabricate various technological devices including gyroscopes, bolometers, low frequency comb drives, high frequency resonators, micromirrors andmetal oxide semiconductor field effect transistors (MOSFETs) [1-8]. These applications are feasible because of the low thermal budget for poly-SiGe films(~450℃) which has proven useful when monolithically integrating Micro/Nano- ElectroMechanical System (M/NEMS) with its driving electronic components in the MEMS-last approach[1,4]. Also, poly-SiGe has a high melting temperature (>900℃), a high tensile strength (~1.60GPa) and a high creep resistance [1]. By alloying Si with germanium (Ge), the stress can be substantially reduced even at lower fabrication temperatures (~650℃ or lower). Improvement in the tensile stress is attributed to a higher crystallinity and also the higher thermal stress associated with a higher germanium fraction [9]. Poly-SiGe possesses good piezoresistive properties and can easily be modulated by changing the doping concentration as well as the germanium fraction[10].The structural components of most MEMS devices are fabricated with micrometre thick poly-SiGe films making surface effect irrelevant. Because of the need for device miniaturization, knowledge of size dependent properties of poly-SiGe becomes sine qua non. With miniaturization, portable and compact devices are fabricated. Such devices consume less power, have high mechanical resonance frequencies and higher quality factors which are particularly important for surface based sensing and detection[10,11].Compared to deposition techniques such as plasma enhanced chemical vapour deposition, evaporation and RF sputtering; low pressure chemical vapor deposition (LPCVD) yields poly-SiGe films of high uniformity, conformability and reproducibility[3,9]. Interestingly, film with low resistivity, high mobility, low surface roughness, and low strain gradient can be deposited by tuning the deposition parameters including deposition temperature, chamber pressure and germanium fraction. For those applications that require high deposition rate and big grain size,increasing deposition temperature can be of immense importance. Previous works[10,13] have suggested that the structures and properties of poly-SiGe ultrathin films are sensitive to the process parameters and that higher deposition rate and larger grain size can be obtained if the substrate temperature and chamber pressure are appropriately tuned. To investigate this, we deposited poly-SiGe films at various substrate temperature and chamber pressure using LPCVD and characterized them using various techniques. This paper describes the influence of deposition temperature and pressure on crystallinity, resistivity, grain size, deposition rate, Hall mobility, carrier concentration and surface roughness of ultrathin poly-SiGe films.
2. Experimental Techniques
2.1. Film Deposition
- Poly-SiGe films were deposited on SiO2/Si(100) substrate using an Applied Materials Centura LPCVD system. The films were deposited by thermal decomposition of pure silane (flow rate of 8 sccm) and germane (10% in Hydrogen, flow rate of 180sccm) with hydrogen flow rate of 500 sccm, diborane flow rate of 11 sccm and heater-shower head spacing of 500 mil. These deposition conditions were selected by grey-Taguchi optimization technique as reported in another publication[14]. Both substrate temperature and chamber pressure were varied (Table 1) to study their influences on some structural and electrical properties of poly-SiGe films. To suppress thickness effects on these properties, the deposition times were adjusted such that the film thicknesses do not exceed 100 ± 5 nm.
|
2.2. Characterization Techniques
- The deposited films were characterized by Scanning Electron Microscopy (SEM, FEI Nova 200), Atomic Force Microscopy (AFM, Multimode Digital Instrument), 4-point probe (KLA-Tencor OmniMap RS75), Hall Effect measurement (Keithley Instruments GmbH) andX-Ray Diffractometery (XRD, PANalytical X’Pert PRO MRD operating in 2θ-ω mode). Using Rutherford Backscattered Spectroscopy (RBS) and Secondary Ion Mass Spectroscopy (SIMS), the germanium fraction and the boron concentration in the films were measured to be ~87% and 3.47 x 1021cm-3, respectively. Details of the electrical measurement techniquesare describedin Asafa et al.[15].
3. Results and Discussion
3.1. Influence of Deposition Temperature
- Deposition temperature contributes about 43% to the changes in the properties of poly-SiGe film leaving 57% for other deposition parameters[see Ref. 14]. The diffraction patterns of ultrathin poly-SiGe films grown under different deposition temperature show an increase in the peak intensity and reduction in thefull-width at-half-maximum (FWHM) (Fig. 1a). The transformation of the diffraction peaks appearing at around 2θ = ~28°, 46° and 53°, correspond to (111), (220), and (311) crystal planes of Si13Ge87, respectively. The diffraction peaks of the single crystalline Si substrate were suppressed by applying ω-offset of 3o following the procedure discussed elsewhere[16]. All the films are polycrystalline as revealed by the significant peak intensities of the crystal planes which is consistent with published studies[17-21]. From these spectra, existence of single peaks at diffraction angles between the expected angles for Ge and Si is noted. This implies that the layers are of a homogeneous material and not built out of large clusters of Ge-rich material embedded in silicon or vice versa. For the deposition temperaturebetween 390oC and 455oC, the XRD peak intensity is greatly enhanced while no significant shift in the lattice constant is observed. The lattice spacing
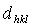 for plane (hkl) is calculated from the well-known Bragg’s equation:
for plane (hkl) is calculated from the well-known Bragg’s equation: 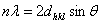 where n/
where n/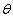 and
and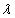 are the diffraction order/angle and the wavelength of x-ray, respectively. For the first order diffraction and given λ = 0.154 nm;
are the diffraction order/angle and the wavelength of x-ray, respectively. For the first order diffraction and given λ = 0.154 nm; 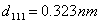 ,
,  and
and  . These values are essentially similar for all the films irrespective of the deposition temperature. The lattice constant a = ~0.557 nm as estimated from
. These values are essentially similar for all the films irrespective of the deposition temperature. The lattice constant a = ~0.557 nm as estimated from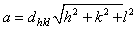 .The influence of deposition temperature on the grain size D was calculated by using Scherrer’s equation, that is: D = 0.9λ/(β*cosθ)[22], where β is the FWHM measured in radians. Each grain size indicated in Fig. 1(b) is an average value based on the FWHM calculated from the 3 peaks. For the range of deposition temperature considered, the grain size increases from ~12 nm to ~33 nm. This enhancement is attributed to grain coalescence and diffusion of adatoms. As a result of the increment, the grain boundary density and the associated defects are expected to decrease[23]. Consequent upon reduction in the carrier scattering at the grain boundaries, the film resistivity reduces from 1.6 to 0.9 mΩcm (Fig. 1b) with corresponding increase in the carrier mobility from ~2.3 to ~6.4 cm2V-1s-1 (Fig. 1c).Whileboron concentration remains at ~3.47 x1021 atoms/cm3, the active carrier concentration reduces from 1.65 x1021 to 1.05 x1021 atoms/cm3. The difference between the effective carriers and the boron concentrations shows that Hall factor is less than unity. Generally, the low value of the Hall mobility may be attributed to the polycrystalline structure of the films. In practice, a structural layer having low resistivity and high mobility is desired for nanoswitches which require low pull-in and pull-out voltages for efficient performance.
.The influence of deposition temperature on the grain size D was calculated by using Scherrer’s equation, that is: D = 0.9λ/(β*cosθ)[22], where β is the FWHM measured in radians. Each grain size indicated in Fig. 1(b) is an average value based on the FWHM calculated from the 3 peaks. For the range of deposition temperature considered, the grain size increases from ~12 nm to ~33 nm. This enhancement is attributed to grain coalescence and diffusion of adatoms. As a result of the increment, the grain boundary density and the associated defects are expected to decrease[23]. Consequent upon reduction in the carrier scattering at the grain boundaries, the film resistivity reduces from 1.6 to 0.9 mΩcm (Fig. 1b) with corresponding increase in the carrier mobility from ~2.3 to ~6.4 cm2V-1s-1 (Fig. 1c).Whileboron concentration remains at ~3.47 x1021 atoms/cm3, the active carrier concentration reduces from 1.65 x1021 to 1.05 x1021 atoms/cm3. The difference between the effective carriers and the boron concentrations shows that Hall factor is less than unity. Generally, the low value of the Hall mobility may be attributed to the polycrystalline structure of the films. In practice, a structural layer having low resistivity and high mobility is desired for nanoswitches which require low pull-in and pull-out voltages for efficient performance. | Figure 1. Influence of deposition temperature on (a) XRD spectra (b) resistivity and grain size (c) carrier concentration and Hall mobility (d) deposition rate and rms roughness of poly-SiGe films |
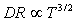 while
while 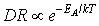 for the surface-reaction limited process, where k (=8.61*10-5 eV/K) is the Boltzmann’s constant[24]. The Arrhenius plot
for the surface-reaction limited process, where k (=8.61*10-5 eV/K) is the Boltzmann’s constant[24]. The Arrhenius plot 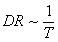 for the temperature-dependent behaviour of poly-SiGe is shown in Fig. 1(d) (slope = -1805). The thermal activation energy isderived to be 160 meV with linear correlation coefficient of 0.9991, an indication of a very good exponential relation. The value of the activation energy is lower than 200 meV calculated for the valence band offset for epitaxial strained Si1-xGex (x = 0.75)[25] and 233 meV estimated for the Si/Si1-xGex/Si single-quantum-well sample grown by molecular-beam epitaxy and annealed at 700oC[26]. The lower value might be due to the polycrystalline structure which requires lower energy due to multidirectional growth compared to the epitaxial films. It also shows that the formation of poly-SiGe requires low energy compared to the electrochemical deposition of platinum which requires ~2 eV/atom[27].With increased deposition temperature, AFM images (Fig. 2) show a slight increase in the root-mean-square (rms) of the surface roughness (Fig. 1d). Although, smoother surface might be expected due to an increased surface mobility of the species[28], the increased rms roughness may be due to increased strain between the film and the substrate as well asbigger grain size due to coalescence(Fig. 1b).For surface based sensing and detection and other applications where surface modification is essential, a low value of surface roughness is desirable.
for the temperature-dependent behaviour of poly-SiGe is shown in Fig. 1(d) (slope = -1805). The thermal activation energy isderived to be 160 meV with linear correlation coefficient of 0.9991, an indication of a very good exponential relation. The value of the activation energy is lower than 200 meV calculated for the valence band offset for epitaxial strained Si1-xGex (x = 0.75)[25] and 233 meV estimated for the Si/Si1-xGex/Si single-quantum-well sample grown by molecular-beam epitaxy and annealed at 700oC[26]. The lower value might be due to the polycrystalline structure which requires lower energy due to multidirectional growth compared to the epitaxial films. It also shows that the formation of poly-SiGe requires low energy compared to the electrochemical deposition of platinum which requires ~2 eV/atom[27].With increased deposition temperature, AFM images (Fig. 2) show a slight increase in the root-mean-square (rms) of the surface roughness (Fig. 1d). Although, smoother surface might be expected due to an increased surface mobility of the species[28], the increased rms roughness may be due to increased strain between the film and the substrate as well asbigger grain size due to coalescence(Fig. 1b).For surface based sensing and detection and other applications where surface modification is essential, a low value of surface roughness is desirable. 3.2. Influence of Chamber Pressure
- Although increasing chamber pressure from 50 to 80 Torr does not influence most properties of poly-SiGe films, it however slightly enhances Hall mobility and deposition rate.Based on the XRD spectra of Fig. 3(a), increasing chamber pressure does not significantly influence thefilm crystallinity as evident from the nearly equal peak intensities and FWHM for the diffraction planes. The resistivity and the grain size (calculated from Scherrer’s formula) remain virtually unchanged within the pressure range considered (Fig. 3b). This implies that chamber pressure is not an appropriate parameter if the crystallinity and the grain size of poly-SiGe films are to be modulated significantly. The carrier concentration increasesvery slightly from ~0.65 x1021 to 1.2 x1021 atoms/cm3 when the chamber pressure is increased from 50 to 70 Torr(Fig. 3c). However, a turning point is observed at a pressure of 70 Torrfollowed by a decrease incarrier concentration. Because the change in the resistivity with pressure is negligible, the Hall mobility is inversely proportional to the carrier concentration (Fig. 3c). Despite similar boron concentrations in the films, this behaviour is expected since the quantity of the effective carriers is influenced by the combined effects of the boron concentration as well assilicon and germane fractions. A higher pressure increases the amount of Si and Ge precursors which subsequently increases the deposition rate [29,30]. Therefore, the deposition rate increases as the chamber pressure goes up (Fig. 3d). However the rms roughness is not affected. It merely decreases from 4.77 nm to 4.57 nm with the difference lying within measurement error. The nearly constant value of rms roughness may be due to the constant grain size (Fig. 3b).
 | Figure 3. Influence of chamber pressure on (a) XRD spectra (b) resistivity and grain size (c) carrier concentration and Hall mobility (d) deposition rate of poly-SiGe films |
 | Figure 4. AFM images of poly-SiGe ultrathin films deposited under chamber pressure of (a) 50 Torr (b) 80 Torr at a constant germanium fraction of 0.87 and boron concentration of 3.57 x 1021 cm-3 |
4. Conclusions
- It is evidenced from this paper that the structural and electrical properties of poly-SiGe films grown by low pressure chemical vapour deposition are strongly influencedby deposition temperature.For films with a germanium fraction of ~87% and a boron concentration of 3.47x1021 atoms/cm3, it is observed that by increasing the deposition temperature from 390 to 455oC; the deposition rate, Hall mobility and resistivity are significantly enhanced. These improvements are due to bigger grain size which reduces grain boundary defectsand subsequent carrier recombination. It is also shown that the thermal activation energy is lower for poly-SiGe than for epitaxial SiGe. Also, increasing chamber pressure from 50 to 80 Torr does not significantly influence the crystallinity, grain size and resistivity while slightly enhance the deposition rate.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
