-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2013; 3(4): 75-89
doi:10.5923/j.nn.20130304.01
Eco-friendly Application of Nano Chitosan in Antimicrobial Coatings in the Textile Industry
K. Vellingiri1, T. Ramachandran2, M. Senthilkumar3
1Department of Fashion Technology, PSG College of Technology, Coimbatore, Tamilnadu, India
2Principal, Karpagam Institute of Technology, L & T Bypass Road, Bodi palayam post, 641 105, Coimbatore
3Department of Textile Technology, PSG College of Technology, 641004, Coimbatore
Correspondence to: K. Vellingiri, Department of Fashion Technology, PSG College of Technology, Coimbatore, Tamilnadu, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
An attempt was made on, chitosan synthesized into nano particles and characterized through Transmission Electron microscope (TEM) and Dynamic light scattering (DLS). The individual size varied from 5 nm to 180 nm respectively in TEM and DLS. Nano chitosan particles were applied on 100% cotton, viscose and polyester material to impart anti microbial finish. The Fourier Transform Infrared spectroscopy (FTIR) and Scanning Electron Microscope (SEM) analysis exposed the embedding of chitosan nano particles. The treated fabrics were tested for anti microbial activity as per AATCC 100-2004 and AATCC 124-2009 and the results show that the demonstrated significant antibacterial activity against S. aureus in both qualitative and quantitative tests. The physical properties of treated and untreated fabrics are also analyzed and reported. The environmental testing included an effluent treatment and recycling and selected heavy metal contents and formaldehyde. A biological method using the sequence Lactobacillus acidophiluswas proposed to determine the viability of reusing contaminated water discharged. Contents of selected heavy metals and formaldehyde have also been tested in Atomic Absorption Spectroscopy (AAS) and UV-Spectrophotometer and reported. Finally these treated fabrics proved that it is durable and eco-friendly process and discharged effluents were also environmental free and cost effective process.
Keywords: Ionic-gelation Technique, Nano Chitosan; Environmental Safety, Biological Treatment, Lactobacillus Acidophilus, Heavy Metals
Cite this paper: K. Vellingiri, T. Ramachandran, M. Senthilkumar, Eco-friendly Application of Nano Chitosan in Antimicrobial Coatings in the Textile Industry, Nanoscience and Nanotechnology, Vol. 3 No. 4, 2013, pp. 75-89. doi: 10.5923/j.nn.20130304.01.
Article Outline
1. Introduction
- Antimicrobial textiles provide the benefits in hygiene, odor control and protection of the fabric from microbial attack, bacterial resistance to the biocides used and their toxic breakdown products in the household and environment have been concerns. Most biocides used on commercial textiles can induce bacterial resistance to these substances, which can lead to increased resistance to certain antibiotics in clinical use[18]. With increasing demand for fresh and hygienic textiles, the consumption of anti microbial is increasing day by day. To produce more and more textile products are necessary to have effective and safe environment. It is essential to have natural and inorganic sources with new technology to produce health and hygienic products11. Bio materials are more complex of different compounds and it depends upon geographical location, age and extraction methods. But their eco-friendly nature and non toxic properties facilitate for medical and health care textiles [15]. In recent research studies have revealed that chitosan is more effective in inhibiting the growth of bacteria than chitosan oligomers[9]. Synthesized nano scale core–shell particles of poly (n-butyl acrylate) cores and chitosan shells applied them to cotton fabrics in a pad–dry–cure process. The antibacterial activities were maintained at over 90% reduction levels after 50 washes[16,17]. To improve antimicrobial durability, chitosan has been cross linked to cotton using chemicals such as dimethylol dihydroxy ethylene urea (DMDHEU), citric acid, 1, 2, 3, 4-Butane tetra carboxylic acid (BTCA) or glutaric dialdehyde .These chemicals, some of which are used in cotton durable press, crosslink chitosan to cotton through hydroxyl groups[6, 12, 19, 25]. Due to very small size of chitosan nano-particles or structures, their surface areas are very high and processes involving nano-particles or nanotechnology become much more efficient than the conventional ones[21,22]. Nanotechnology also holds big promise for textile related activities like smart and intelligent textiles, better finishing treatments and technical textiles. Due to the Antimicrobial action of the amino group at the C-2position of the glucosamine residue, chitosan is also known to be an antimicrobial polysaccharide. The carboxylic groups in the chitosan structure were used as active sites for its fixation onto cotton fabrics[1]. The ability of chitosan to immobilize microorganisms derives from its poly cationic character. Its protonised amino groups block the protein sequences of microorganisms, thus inhibiting further proliferation. Chitosan binds to the negatively charged bacterial surface disrupting the cell membrane and altering its permeability. This allows materials to leak out of the bacterial cells resulting in cell death. Chitosan can also bind to DNA inside the cell inhibiting mRNA and hence protein synthesis. Nano-size materials are able to enhance the physical properties of bulk materials. This characteristic is reflected in conventional textiles in areas such as anti-microbial properties, water-repellency, soil-resistance, anti-static, anti-infrared and flame retardant properties, dye ability, and strength of textile materials[13, 10, 29-32]. Nanotechnology can provide high durability for fabrics, because nano-particles have a large surface area to volume ratio and high surface energy, thus presenting better affinity for fabrics and leading to an increase in durability of the function. In addition, a coating of nano-particles on fabrics will not affect their breathability or hard feel. Therefore, the interest in using nano technologies in the textile industries is increasing[23]. The formation of nano chitosan depended on the TPP concentration; when the TPP Concentration was excessively low or high, it failed to react with the chitosan to form nano particles[20]. Antimicrobial finish provides the various benefits of controlling the infestation by microbes protect textiles from staining, discoloration, and quality deterioration and prevents the odor formation. Anti-microbial agents can be applied to the textile substrates by exhaust, pad-dry-cure, coating, spray and foam techniques. The application of the finish is now extended to textiles used for outdoor, healthcare sector, sports and leisure. The application of nano finishes has also been growing to obtain better level of performance properties. Ecology and Fashion are also the driving factors for the adoption of these innovative technologies[24]. After launderings nano finished fabric samples exhibit superior performance. Even after 50 home launderings, percent reduction in bacteria present remains the same100% in these fabric samples. On the other hand, the durability of the treatment reduces considerably with fabrics treated with normal finish5. Implementation of nano materials improve properties and gain newer multi functionalities to the fabrics [7]. In case of discharged effluents, collected from desized, scoured, bleached and anti microbial finished were treated with aerobic biological treatment (Lactobacillus acidophilus culture). Bacteria offers a cheaper and environment friendlier alternative for color removal in textile effluents. Biological treatment has been effective in reducing dye house effluents and when used properly has a lower operating cost than other remediation process [14]. The determination of the best conditions of preparation of a (tentatively) probiotic starter culture that might be suitable for cheese making composed solely of Bifidobacterium lactis Bo and Lactobacillus acidophilus Ki is critical if a consistently reliable acid production is to be achieved, especially because bifidobacteria have stringent requirements for growth[2]. A satisfactory result can be achieved with aerobic biological treatment. Since the scoured effluents contribute more COD load in the overall effluent from the industry, the present study is advantageous in the cost reduction of ETP. Inorganic matter may cover heavy metals, nutrients (Antimony, arsenic, barium, cadmium, chromium, lead, mercury, selenium and zinc), pH, alkalinity, chlorides, sulfur, and other inorganic pollutants. Gases such as carbon dioxide, nitrogen, oxygen, hydrogen sulfide, and methane may be present in wastewater (Lee and Lin, 2000). Wastewaters are normally treated by a combination of physical-chemical and biological operations. However, it is possible to treat waste waters solely with physical-chemical methods (Droste, 2004)[4]. The COD and BOD of treated effluents were reduced significantly to a greater due to the biological treatment process for which the effluent is passed [8]. Physicochemical treatment of the wastewater yielded good solid reduction after filtration, high BOD and COD reduction, high nitrate and phosphate reduction, thus preventing eutrophication due to these undesirable nutrients and also maximum bacteria kill. The wastewater could therefore be discharged safely without the fear of pollution[3]The reuse of effluents will lead to water saving, reduced energy consumption, and lower effluent treatment costs[15]. Heavy metal content of fabric was defined by section 3.3.5 of the Good Environmental Choice Australia standard 19-2004 Textile Products[20.26, 27]As quoted by many researchers in the nano chitosan particles in the field of medical textile application, an attempt has been made to study the characteristics of nano chitosan particles to impart antimicrobial finish on 100% cotton, viscose and polyester fabrics and testing the performance of antimicrobial effect, characteristics of discharged water and environmental load are reported hereunder.
2. Materials and Methods
2.1. Materials
- The plain weave grey fabrics of 100% cotton, viscose and polyester were chosen. The cotton fabric was procured with 9 tex in warp count, 9.4 tex in weft count, 40 ends / cm, 31 picks / cm and 71 g /m2. The viscose was procured with 20.2 tex in warp, 22.5 tex in weft, 18 ends / cm, 19 picks / cm and 84 g /m2. The polyester was procured with 6.8 tex in warp, 7.1 tex in weft, 50 ends / cm, 26 picks/cm and 78g/m2. Commercial chitosan has low molecular weight (powder in 177-420 microns) was used as antimicrobial agent and degree of deacetylation is 75% and was procured from M/s Cochin Refineries Company, Government of India, Kerala. Sodium tri poly phosphate (STPP) AR grade concentration 0.5% on weight of material (owm) was used as binder and acetic acid was used as dissolving agent and deionized water as a medium.
2.2. Methods
2.2.1. Synthesis of Nano Chitosan Particles
- The commercial chitosan 0.5% concentration was dissolved in 1.5% w/v acetic acid and this is taken to sonicator for nano conversion process. The sonication process carried out for 20 minutes at 45 watts; using (make & model) a B. Braun Labsonic L sonicator. A 0.5% w/v concentration of sodium tripolyphosphate (STTP) solution was also prepared with distilled water. The STTP solution was added drop wise with a syringe to chitosan solution while stirring, followed by sonication for 20 minutes. The resulting suspension was subsequently centrifuged at 10,000 rpm for 15-20 minutes. The pellets obtained were re-suspended in deionized water by sonication and centrifuged.
2.2.2. Characterization of Nano Chitosan Particles Using TEM and XRD
- The individual particle size of nano synthesized chitosan was assessed by the JEOL JEM 2100 High Resolution Transmission Electron Microscope (HRTEM), enables to view lattice resolution of 0.14 nm and point-to-point resolution of 0.19 nm and with 200 kV acceleration voltage and Gatan Orious CCD camera for image capturing. The dried samples are analyzed to determine the individual size The crystallinity of the nano particles was determined by XRD using a SHIMADZU–XRD 6000 advanced X-ray difractometer equipped with a CuKα radiation, λ=1.5406 A0 source (applied voltage 30kV, current 30mA).The dried nano chitosan particles were deposited as a randomly oriented powder on to a plexiglass sample container, and the XRD patterns were recorded at angles between 100–800, with a scan speed of 5 /minute, sampling pitch of 0.020, and preset time 0.24 second. The crystalline domain Diameters (D) were obtained from XRD peaks according to the Debye-Scherrer’s equation [1].
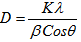 | (1) |
2.2.3. Coating of Nano Chitosan Particles on Fabrics
- Optimal concentrations (0.5%) of nano Chitosan particles was chosen for antimicrobial finishing .This was given to the fabrics after desized, scoured and bleached process as per standard methods and using exhaustion method with the following composition. 8% citric acid was used as cross-linking agent. The fabric was kept immersed in the solution (MLR 1: 20) for 30 minutes at 50ºC in water bath. After finishing, the fabrics was removed, squeezed and dried at 80ºC in the oven for 5 minutes and then cured at 120ºC for 2 minutes.
2.2.4. Determination of Antibacterial Efficacy
- Test Organism used is Escherichia coli ATCC (American Type Culture Collection) 11229 and - Staphylococcus aureus ATCC 6538 (antimicrobial susceptibility data) Initial inoculum is E. coli – 2.7 × 109 cfu/ml and S. aureus – 2.4 ×109 cfu /ml and as of méthodologie. 5.0 cm diameter of the treated fabric was taken and it was immersed in sterile AATCC broth with 0.1 ml inoculums of each culture (Staphylococcus aureus and Escherichia coli) and left overnight at 37C in shaker. Control was also maintained with untreated fabric. AATCC broth with the fabric was taken for appropriate dilutions. The broth was spread plated on AATCC agar plates. The plates were incubated at 37C for 24 hrs and after incubation results were interpreted. The percentage reduction of bacteria after incubation was calculated by the following formula [2].
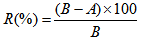 | (2) |
2.2.5. Determination of Wash Fastness of Nano Coated Fabrics
- The wash fastness of nano chitosan coated fabrics was tested as per AATCC method 61(1996) test no.2A using Atlas Launder-o-meter LEF instrument.
2.2.6. Physical Properties of Nano Chitosan Coated Fabrics
- The physical properties of woven fabrics such as fabric mass ASTM D 3776: 2009, tensile strength ASTM D5035:2006, tearing strength ASTM D1424:2009, and air permeability ASTM D737 2008 are important factors in determining the fabric properties. Both untreated and treated fabric samples were evaluated after conditioning the specimens at 65% RH and 27 ± 2ºC for 24 hours by bringing them to approximate moisture equilibrium in the standard atmosphere for preconditioning textiles as directed in Practice D1776 in an environmental chamber (ASTM2008).
2.2.7. Determination of Chemical Group of Nano Coated Fabrics Using FTIR
- FTIR spectrometer was obtained using a NICLET- IS10 spectral range of 4000—1000 cm-1.The nano treated chitosan samples and untreated samples were tested for its chemical group’s presence.
2.2.8. Determination of Dispersion of Nano Chitosan Particles on Coated Fabrics Using SEM
- The fabric samples treated with the nano-particles were mounted using double sided tape on a specimen stub and coated with gold in a sputter coater and then examined with a Jeol Model JSM-6360.
2.2.9. Formation and Application of Lactobacillus Acidophilus (Organism) on Effluent Treatment
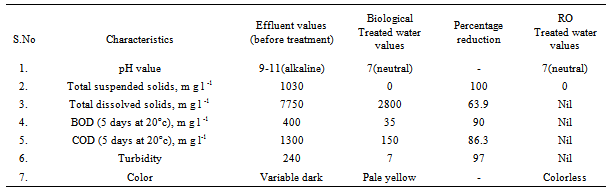
- The various bio materials required for the formation of culture in 12% (v/v) of A, B, C, D, E & F (by products of milk). The above bio materials were taken in the equal ratio and mixed with water in the ratio of 1:1 and were closely packed and stored in air tight containers at room temperature for 28 days. The appearance of biomaterial at the initial and final stages was shown in Figure 5a & 5b respectively. The collected effluents sample (desized, scoured, bleached and antimicrobial finished) was ozonized at pH 7 in ambient condition. After testing the culture in laboratory, it is observed that curdling of milk marked the growth of lactobacillus acidophilus as shown in Figure 5c.The effluents from desized, scoured, bleached and anti microbial finished was collected from the discharge unit of the textile wet processing unit M/s. sri suryodhayaa processing private limited company, Erode, Tamilnadu, India. The effluent sample of 50 litters was collected directly into containers. After adding 10 ml of manganous sulphate solution to fix the dissolved oxygen, it was stored at room temperature. The collected effluent and treated effluent characteristics was given in Table 4.
2.2.10. Determination of Heavy Metal Content and Formaldehyde Content in Nano Chitosan Coated Fabrics
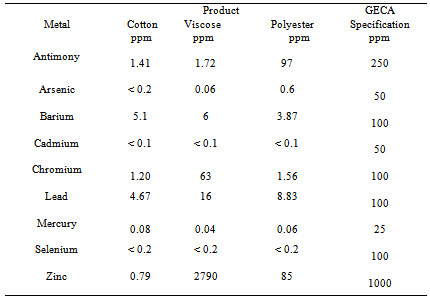
- The scope of environmental testing included an effluent treatment and recycling and selected heavy metal content and formaldehyde of anti microbial treated fabrics. Selected heavy metal contents tested in Analogous DIN 38414 S7 based on DIN 38406-E29 by means of AAS/ICP MS (Atomic Absorption Spectrophotometer/ Integrated Coupled Plasma Mass Spectroscopy) and formaldehyde have been tested as per BS EN ISO 14184 PART-1-2011and the test results were given in Table 5. Heavy metal content of fabric was defined by section 3.3.5 of the Good Environmental Choice Australia standard 19-2004 Textile Products.
3. Results and Discussions
3.1. Synthesis and Characterization of Nano Chitosan Particles
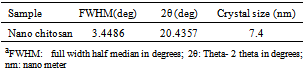
- A TEM image of the prepared nano chitosan particles was shown in Figure 1 (a) and (b).The Nano chitosan particles are spherical in shape with a smooth morphology. The diameter of nano chitosan particles are found to be is in the range of 5-180 nm by scale bar in TEM machine.The XRD spectra patterns of nano chitosan particles obtained using ionic gelation technique is shown in Figure 2 and Table 1. The spectra shows well defined peaks typical of chitosan the crystallinity structure of chitosan, the distinctive nano chitosan particles peaks are at 2 theta0. The XRD patterns of sample chitosan showed three strongest distinctive chitosan peaks, the distinctive chitosan peaks were at 20.4, 26.4 and 29.5. The mean crystallite size of a powder sample was estimated from the full width at half-maximum (FWHM) of the diffraction peak according to the Scherrer’sequation. It suggests the formation of chitosan nano particles.
|
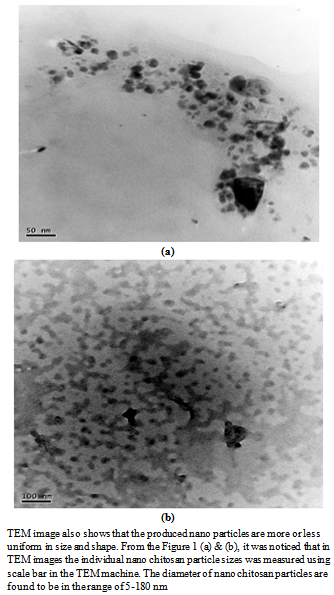 | Figure 1. TEM images of nano chitosan particles at (a) 50 nm scale, (b) 100 nm scales |
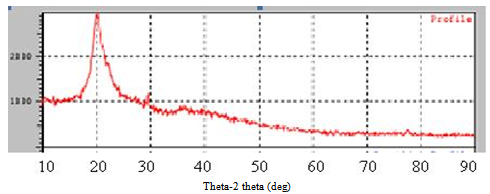 | Figure 2. X-ray powder diffractometry (XRD) pattern of nano particles of chitosan. The crystalline domain Diameters (D) were obtained from XRD peaks according to the Debye- Scherrer’s equation (1) |
3.2. Effect of Nano Chitosan Coating on Antimicrobial Activity of Fabrics
3.2.1. Bacterial Reduction of Nano Coated Fabrics
- The nano chitosan treated fabrics at optimal concentration (0.5%) and the bacterial reduction as R tested are given in Figure 7a, 7b & 7c. The test was carried out with Staphylococcus aureus and Escherichia coli. From the Figure 7a, 7b & 7c it was noticed that the nano chitosan treated fabrics of cotton, viscose and polyester against wash test (AATCC124 2011). This proves that nano chitosan particles treated fabrics have anti microbial effect up to 55 washes and this is the evidence of durable finish.
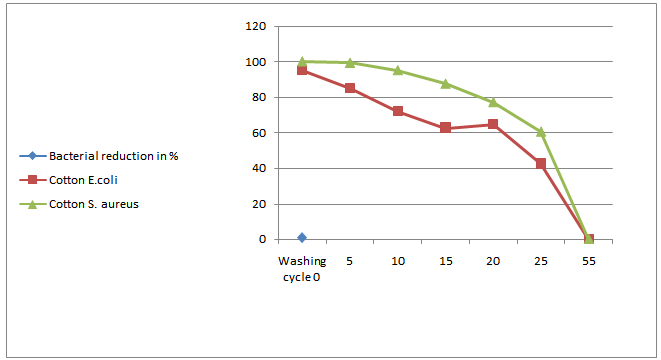 | Figure 7a. Number washes vs. percentage graph of nano chitosan coated cotton fabric |
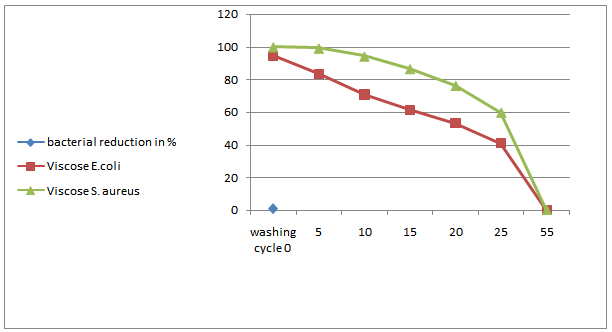 | Figure 7b. Number washes vs. percentage graph of nano chitosan coated viscose fabric |
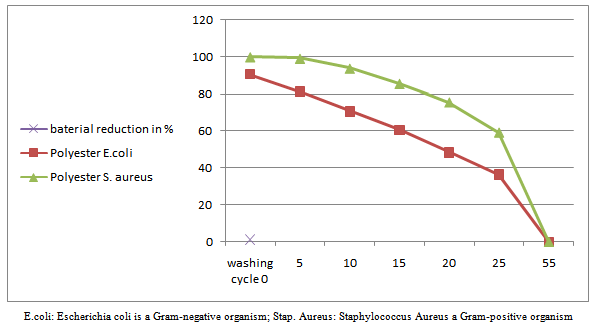 | Figure 7c. Number washes vs. percentage graph of nano chitosan coated polyester fabric |
3.2.2. Physical Properties of Nano Chitosan Coated Fabrics
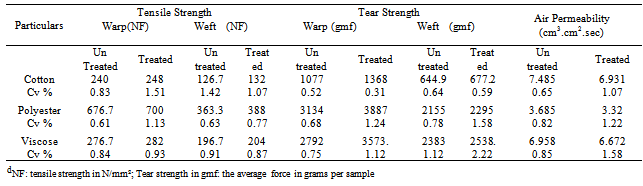
- The Physical properties of cotton, viscose and polyester fabrics were not deteriorated after finished with nano chitosan particles and shown in Table 2.From the Table 2, it can be found that from ANOVA p value was ≤ 0.05, the mechanical properties of cotton fabrics did not detetoriate after finished with nano chitosan particles. In the case of tear strength test both in warp way and weft way was improved from 12% to 20% when compared to untreated fabrics. Finally air permeability test is concerned there is 7-10% improvement in the case of cotton and viscose fabrics and slight decrease of 6% in the case of polyester fabrics.
|
3.2.3. Assessment of Chemical Groups Present in the Nano Chitosan Coated Fabrics
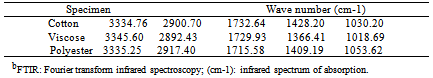
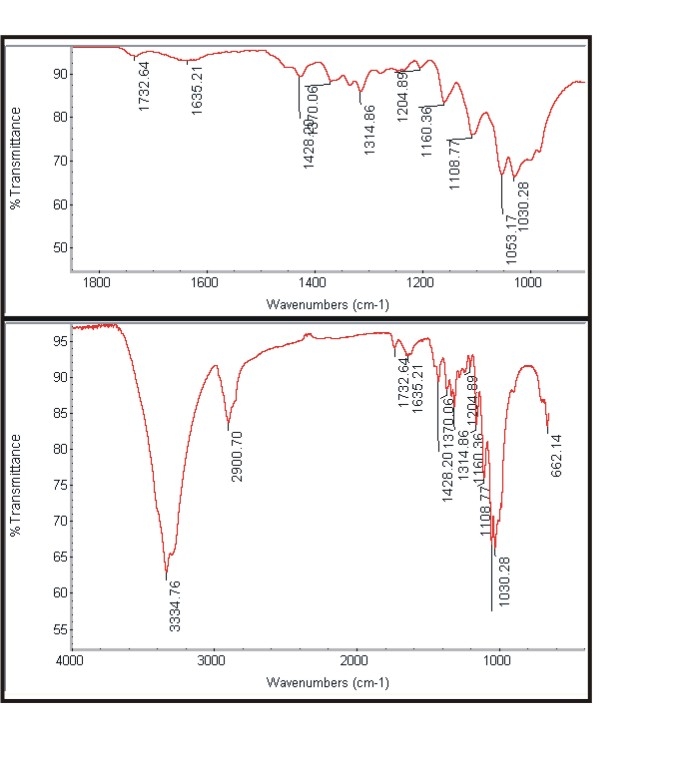
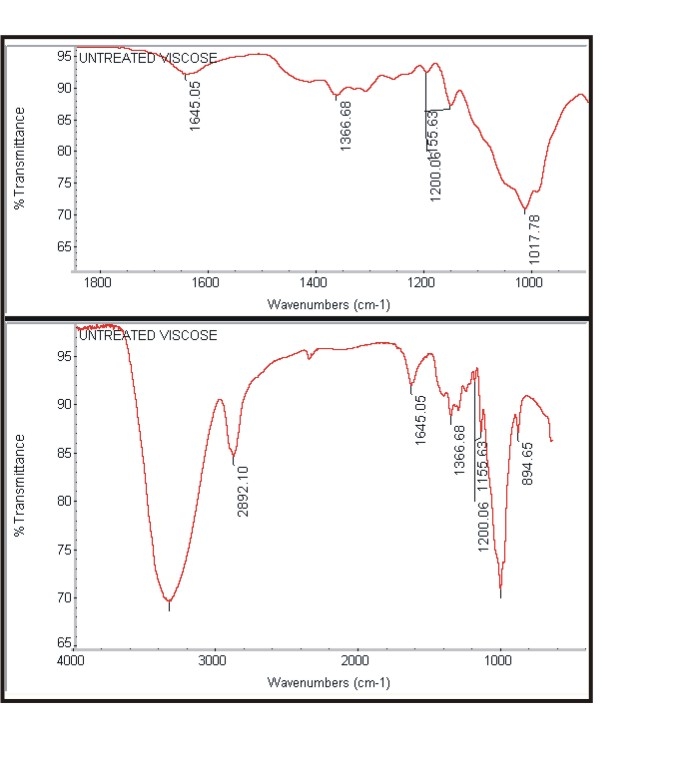
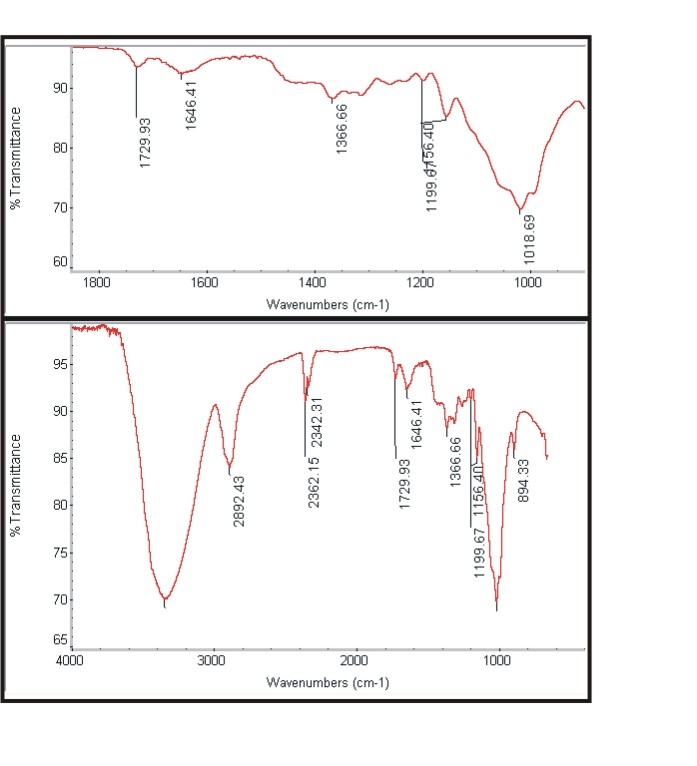
- The Figure 3a and Figure 3b shows the FTIR spectra of untreated cotton fabric sample and treated cotton sample of the spectrum of interference pattern obtained for the nano chitosan particles obtained by the synthesis ionic gelaion technique. Similarly the Figure 3c and Figure 3d shows the FTIR spectra of untreated polyester and treated polyester fabric sample and Figure 3e and Figure 3f shows the FTIR spectra of untreated viscose and treated viscose fabric sample and Table. 3 show the infrared spectrum of absorption of above said samples. From the IR spectrum analysis from Table 3, it is evident that nano chitosan particles are reacting differently with OH groups of cotton and viscose fabrics via `H’ bonding. In the case of polyester nano chitosan particles are reacting differently with ROOC (teraphthalates) group. The -OH stretching vibrations of specimen treated with chemicals could be measured using FTIR and they are expressed in terms of wave number. The - OH stretching vibration of cotton in the presence of chitosan derived from the spectra is presented. The data indicate the OH vibrations of cotton which occur at higher wave number when treated with normal chitosan and at relatively lower wave number when treated with nano chitosan particles. This observation suggests that chitosan reacts strongly with the - OH groups of cotton thus making stronger bonds.
|
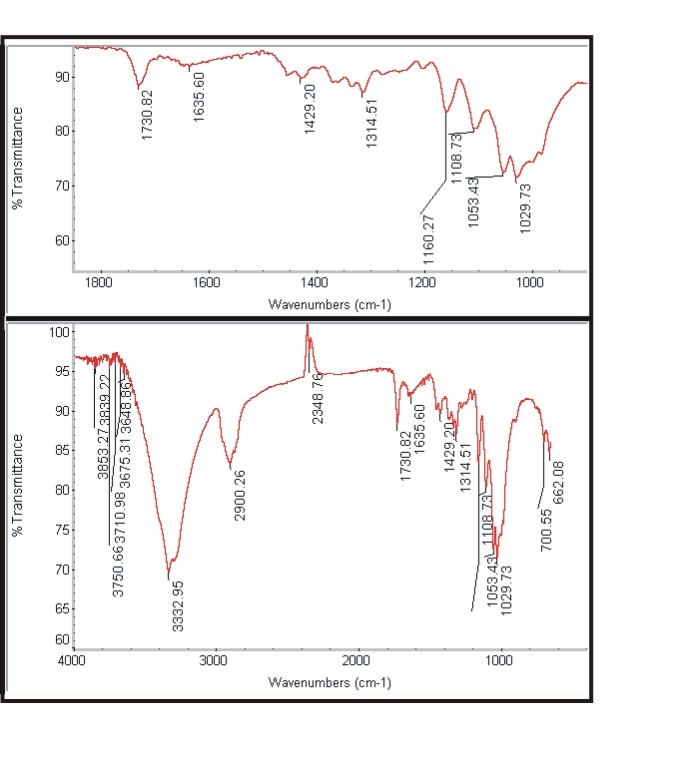 | Figure 3a. Spectra of untreated cotton |
 | Figure 3b. Spectra of treated cotton |
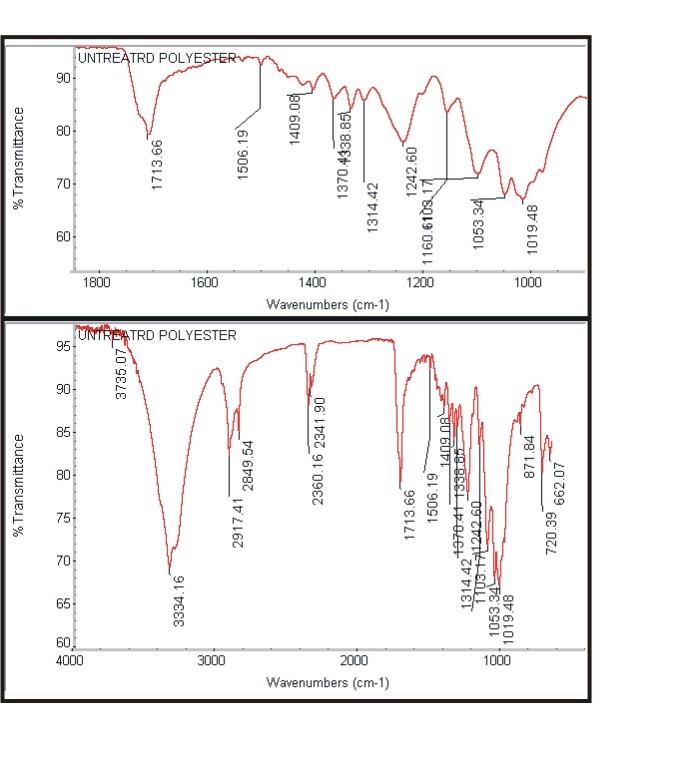 | Figure 3c. Spectra of untreated polyester |
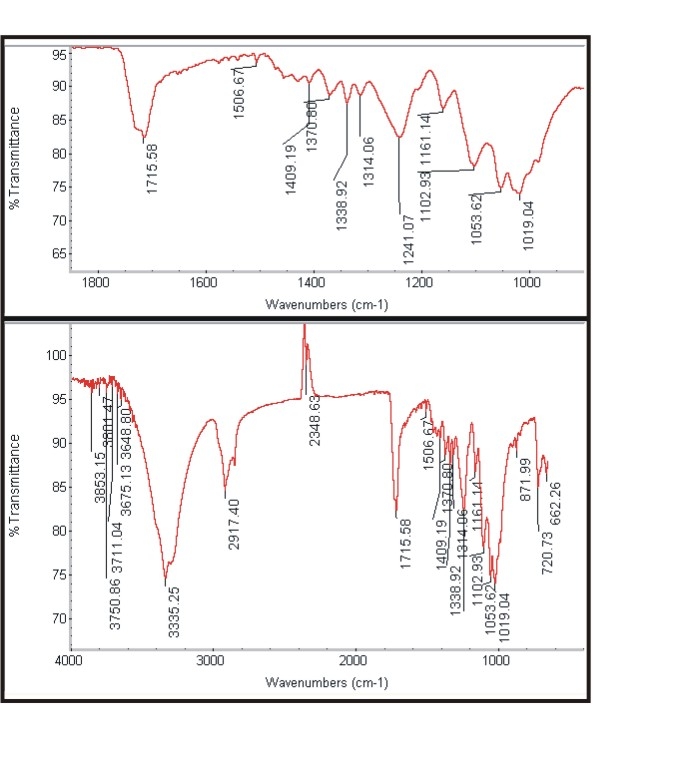 | Figure 3d. Spectra of treated polyester |
 | Figure 3e. Spectra of untreated viscose |
 | Figure 3f. Spectra of treated viscose |
3.2.4. Dispersion of Nano Chitosan Coated Particles in the Cotton Fabrics
- Figure 4(a) shows the SEM micrograph of the untreated 100% cotton woven fabric. Figure 4(b) SEM micrograph shows the nano treated chitosan particles on cotton samples. The nano particles are well dispersed on the fibre surfaces and are finely dispersed and embedded.
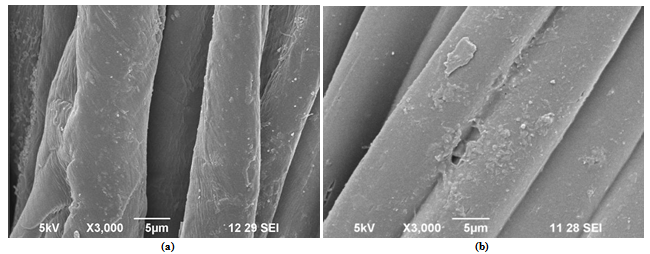 | Figure 4. Photographs of (a) untreated 100% cotton fabric, (b) chitosan Treated 100% cotton fabrics with different magnifications |
3.3. Effect of Lactobacillus Acidophilus Treatment for Effluents
- Effluent samples collected from the industry were kept for 4 hours to reach room temperature. A pH value of 7 was maintained by adding hydrochloric acid to the effluent. The quantity of effluents was measured and the bactericidal lactobacillus acidophilus was added to effluents in the ratio of 1:100 and the samples were stored for 4 days. Figure 5a, 5b and 5c shows the culture formation at the beginning, final and formation of lactobacillus acidophilus respectively.
 | Figure 5(a). Culture Formation – Starting Stage |
 | Figure 5(b). Culture Formation – Final Stage |
3.3.1. Effluents Characteristics with Lactobacillus Acidophilus (Culture) Treatment
- All samples were analyzed as described in the Standard Methods for the Examination of Water and Wastewater and Standard Methods for water and effluent analysis (APHA, 1995; Ademoroti, 1996a).They were preserved to inhibit bio degradation and then analyzed for their characteristics. Table 4 shows Characteristics of the effect of biological treatment on textile effluent treatment before and after and Figure 6a to 6f- show graphically the effect of biological treatment on textile effluent. It is evident from the results as shown in Figure 6a to 6f, that there is an improvement in the effluent characteristics by biological treatment and RO treatment. The color of the effluent was brownish black. The coagulation and flocculation helps to remove color of the effluent. The pH of the raw effluent is very high as the incoming effluent is highly alkaline in nature. The pH correction is done with the help of Hydrochloric acid (HCl) and brings down to neutral which is favourable pH for biological treatment. TDS are composed mainly of carbonates, bicarbonates, chlorides, phosphates and nitrates, calcium, magnesium, potassium and manganese, organic matter salts and other particles.TDS detected could be treated effluent. TDS detected could be attributed to the high color from the various dyestuffs being used in the textile mills. The higher values of COD and BOD in raw effluent attributed to the presence of chemical substances and breakdown of raw material used for preparation of fibre respectively. The COD and BOD of treated effluents were reduced significantly to a greater extent due to the biological treatment process. Evidence from this research suggests that biological color removal of textile wastewaters is sufficient to meet a required level.
|
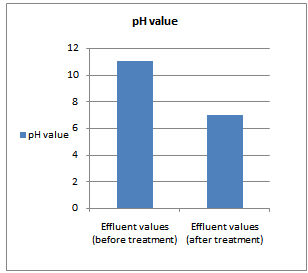 | Figure 6a. pH value before and after treatment |
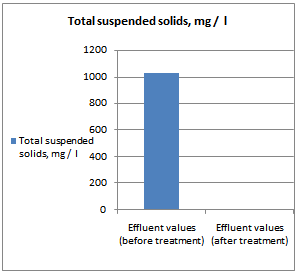 | Figure 6b. TSS value before and after treatment |
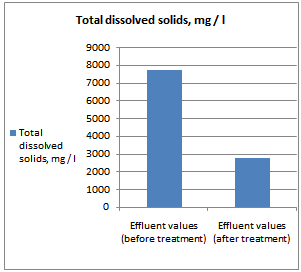 | Figure 6c. TDS value before and after treatment |
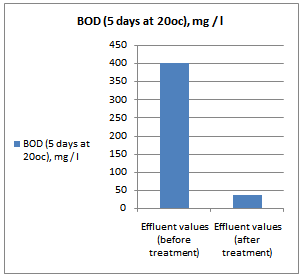 | Figure 6d. COD value before and after treatment |
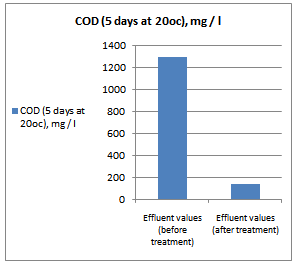 | Figure 6e. COD value before and after treatment |
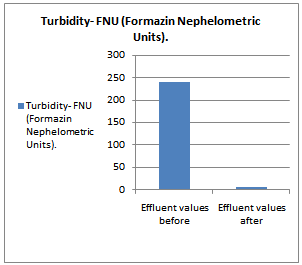 | Figure 6f. Turbidity value before and after treatment |
3.4. Heavy Metal and Formaldehyde Content
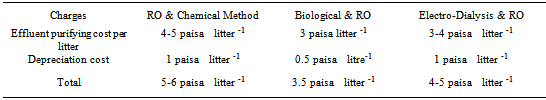
- Selected heavy metal contents and formaldehyde have been tested in Atomic Absorption Spectrophotometer and Integrated Coupled Plasma Mass Spectroscopy and the test results were compared with Good Environmental Choice Australia Standards (GECA) given in Table 5a and Table 5b respectively.With the exception of Antimony the heavy metal content was below the detection limit. Nevertheless with all textile products, the heavy metal content was below the specification. The heavy metal content of all fabric samples was well below the Good Environmental Choice Australia specification. All anti microbial treated fabrics can be classified as having low heavy metal content. This is the evidence of eco-friendliness of all the samples 3.5. CostThe cost comparison between the different methods was given Table 6.This cost was on practical based figures and not the theoretical based and collected from Tirupur district, Tamilnadu, India based effluent industries during the year 2008. It was concluded that biological method for treating textile effluent is economical
|
|
|
4. Conclusions
- The following are the observations made by ionic gel method preparation of chitosan nano particles and antimicrobial application on 100% cotton, viscose and polyester fabrics: The nano chitosan particle conversion was formed and supported by TEM (individual particle varies from 5 nm to 180 nm). Antimicrobial treatment of the treated samples was carried out and the exhaustion method was used to apply the antimicrobial agent on the 100% cotton, viscose and polyester fabrics. Antimicrobial efficacy against staphylococcus Aureus and Escherichia Coli after soap washing was tested and is proved that cotton fabric provided effective antimicrobial activity against both gram positive and gram negative organisms when compared to viscose and polyester fabrics. The effect of antimicrobial coating on the textile properties such as tensile strength, tear strength and air permeability moisture resistance, stiffness resistance, tensile resistance, and abrasion property were also measured and results lie in the acceptable range without much alteration of the fabric properties.Wash durability test carried out with the tested fabrics showed that the significant antimicrobial activity was actively retained in the chitosan nano particles treated fabrics up to 45-50 washes even after repeated wash cycles. This is because of better bound of nano chitosan particles materials with fibre structure. Nano chitosan particles treated fabric physical properties like tensile strength, tear strength and air permeability test both in warp way and weft way was improved from 12% to 20% when compared to untreated fabrics. This may be due to nano nature of material. Important properties of all FTIR spectral are that the spectra of untreated fabric can be especially observed except of the fabric treated by ionic gel technique. A satisfactory result can be achieved with aerobic biological treatment and polishing treatment. Since the dye house effluents contribute more COD load in the overall effluent from the industry, the present study is advantageous in the cost reduction of ETP. The COD and BOD of treated effluents were reduced significantly to a greater due to the biological treatment process for which the effluent is passed. Bacteria offers a cheaper and environment friendlier alternative for color removal in textile effluents. Biological treatment has been effective in reducing dye house effluents and when used properly has a lower operating cost than other remediation process. The reuse of effluents will lead to water saving, reduced energy consumption, and lower effluent treatment costs. This research study analyzes a method which probably is more convenient and provides durable eco-friendly antimicrobial property and environmental safety for everyday clothing, hygiene products, and military clothing. The traditional method exigency in providing durable treatment leads to the development in this area. This research study will also be useful to different application areas. The increased popularity of antimicrobial functional textiles in various application areas means that people are looking forward for a feasible, easy, and durable process that can be carried out in the textile processing plants, creating more durable property.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML