-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2013; 3(3): 62-74
doi:10.5923/j.nn.20130303.06
Semiconductor Nanomaterials, Methods and Applications: A Review
Sagadevan Suresh
Department of Physics, Sree Sastha Institute of Engineering and Technology, Chembarambakkam, Chennai, 600123
Correspondence to: Sagadevan Suresh, Department of Physics, Sree Sastha Institute of Engineering and Technology, Chembarambakkam, Chennai, 600123.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
When the size of semiconductor materials is reduced to nanoscale, their physical and chemical properties change drastically, resulting in unique properties due to their large surface area or quantum size effect. Currently, semiconductor nanomaterials and devices are still in the research stage, but they are promising for applications in many fields, such as solar cells, nanoscale electronic devices, light-emitting nano devices, laser technology, waveguide, chemicals and biosensors. Further development of nanotechnology will certainly lead to significant breakthroughs in the semiconductor industry. This paper deals with the some of the current initiatives and critical issues in the improvement of semiconductors based on nanostructures and nanodevices.
Keywords: Semiconductors, Nanomaterials, Solar Cells, Light Emitting Nano Devices
Cite this paper: Sagadevan Suresh, Semiconductor Nanomaterials, Methods and Applications: A Review, Nanoscience and Nanotechnology, Vol. 3 No. 3, 2013, pp. 62-74. doi: 10.5923/j.nn.20130303.06.
Article Outline
1. Introduction
- A semiconductor is a material that has an electrical conductivity between a conductor and an insulator. In semiconductors, the highest occupied energy band, the valence band is completely filled with electrons and the empty next one is the conduction band. The resistivities of the semiconductor can be altered by up to 10 orders of magnitude, by doping or external biases. In the case of conductors, that have very low resistivities, the resistance is difficult to alter, and the highest occupied energy band is partially filled with electrons and the insulator has extremely high resistivities. It is difficult to alter the resistivity through doping or external fields and the bandgap between the valence band and the conduction band is large. In a metallic conductor, the current is carried by the flow of electrons. In semiconductors, current can be carried either by the flow of electrons or by the flow of positively-charged holes in the electron structure of the material. In the past 10 years, nanomaterials with diameters in the range of 1-20 nm, have become a major interdisciplinary area of research interest and their extremely small feature size has the potential for wide-ranging industrial, biomedical, and electronic applications. Surfaces and interfaces are very important for nanomaterials, but in the case of bulk materials, a relatively small percentage of atoms will be at or near a surface or interface. In nanomaterials, the small feature size ensures that many atoms, perhaps half or more in some cases, will be near the interfaces. Surface properties, such as energy levels, electronic structure, and reactivity can be quite different from interior states, and give rise to quite different material properties. Nanocapsules and nanodevices may present new possibilities for drug delivery, gene therapy, and medical diagnostics. In 1991, S. Iijima[1] reported the first observation of carbon nanotubes. Carbon nanotubes have been shown to have unique properties, stiffness and strength, higher than any other material. Carbon nanotubes are reported to be thermally stable in vacuum up to 2800°C, to have a capacity to carry an electric current a thousand times better than copper wires, and to have twice the thermal conductivity of diamond. Carbon nanotubes are used as reinforcing particles in nanocomposites, but also have many other potential applications. They could be the basis for a new era of electronic devices, smaller and more powerful than bulk materials. The nanocomputer was already made based on carbon nanotubes. Materials having sizes in the range of a nanometer scale have unique properties than bulk materials.Recently there has been substantial interest in the preparation, characterization and application of semiconductor nanoparticles that play a major role in several new technologies. When the size of semiconductor materials is reduced to nanoscale, their physical and chemical properties change drastically, resulting in unique properties due to their large surface area or quantum size effect. The conductivity of the semiconductor and its optical properties (absorption coefficient and refractive index) can be altered. Semiconductor nanomaterials and devices are still in the research stage, but they are promising for applications in many fields, such as solar cells, nanoscale electronic devices, light-emitting diodes, laser technology, waveguide, chemical and biosensors, packaging films, superabsorbents, components of armor, parts of automobiles, and catalysts. Further development of nanotechnology will certainly lead to significant breakthroughs in the semiconductor industry. Semiconductor devices include the various types of transistors, solar cells, many kinds of diodes including the light-emitting diode, the silicon controlled rectifier, and digital and analog integrated circuits. Some of the semiconductor nanomaterials such as Si, Si-Ge, GaAs, AlGaAs, InP, InGaAs, GaN, AlGaN, SiC, ZnS, ZnSe, AlInGaP, CdSe, CdS, and HgCdTe etc., exhibit excellent application in computers, palm pilots, laptops, cell phones, pagers, CD players, TV remotes, mobile terminals, satellite dishes, fiber networks, traffic signals, car taillights, and air bags. The aim of this review is to overview and highlights the applications of semiconductor nanomaterials and synthetic methods. Most semiconducting materials, such as the II-VI or III-VI compound semiconductors show quantum confinement behavior in the 1-20 nm size range. Herein we describe and discuss the current use of semiconductor nanomaterials and their applications.
2. Introductions to Nanoscience and Nanotechnology
- In the past few decades, nanoscience and nanotechnology have been making significant progress and their effect on every field has been truly acknowledged in the world. Therefore, in the 21st century, their strategic position has already been established. The study of nanomaterials and nanostructures is a field with the earliest start that has obtained rich achievements. Nanomaterials and nanostructures play the most important supporting role for applications of nanoscience and nanotechnology in the field of fabrication, such as information & techniques, energy sources, environment, health and medical treatments. Most countries are involved in the study of nanomaterials and nanostructures in a very remarkable way, such as the development of the front fields of nanoscience and nanotechnology, nanoelectronic technologies and devices, nano or microfabrication techniques, nanobiotechnology, nanomedical diagnosis techniques, nanoenvironmental monitoring and treatment techniques. The continuation of the indepth study of nanomaterials and nanostructures are placed in an extremely important position. In the investment for the study of nanoscience and nanotechnology, the actual investment for nanomaterials and nanostructures occupies 49%. Now, the motivational power for the study of nanomaterials and nanostructures is mainly National Strategy Requirements and enhancements of the national competitive ability in the scientific and technological fields. In addition, the (indepth) study of nanomaterials and nanostructures is an important source for establishing new principles, new techniques and new methods, thereby potentially leading to breakthroughs in great scientific problems. At the same time, the nanomaterial market is also a native power for the development of nanomaterials. It will stimulate and promote the development of nanomaterials and nanostructures. Recently there has been substantial interest in the preparation and characterization of materials consisting of particles with dimensions in the semiconductor nanocrystalline materials[2-4].One factor driving the current interest in nanoparticle research is the perceived need for further miniaturization of both optical and electronic devices[5,6].There are practical constraints associated with current technologies; lithographic methods cannot at present be used with a resolution much less than ca. 200 nm. Most semiconducting materials such as the II/VI or III/VI compound semiconductors, show quantum confinement behavior in the 1-20 nm size range, a smaller size than can be achieved, using present lithographic methods.
3. Semiconductor Nanoparticles
- Semiconductor nanocrystals (NCs) are made from a variety of different compounds. They are referred to as II-VI, III-V or IV-VI semiconductor nanocrystals, based on the periodic table groups into which these elements are formed. For example, silicon and germanium are group IV, GaN, GaP, GaAs, InP and InAs are III-V, while those of ZnO, ZnS, CdS, CdSe and CdTe are II-VI semiconductors.
4. Classifications of Semiconductor Nanostructures
- In nanocrystalline materials, the electrons are confined to regions having one, two or three dimensions (Figure 1) when the relative dimension is comparable with the de Broglie wavelength. For a semiconductor like CdSe, the de Broglie wavelength of free electron is around 10 nm. The nanostructures of semiconductor crystals having the z direction below this critical value (thin film, layer structure, quantum well) are defined as 2D nanostructures. When the dimension both in the x and z direction is below this critical value (linear chain structure, quantum wire) the nanostructures are defined as 1D and when the y direction is also below this threshold (cluster, colloid, nanocrystal, quantum dot) it is referred to as 0D.
4.1. Zero Dimensional (0D) Nanostructures
- In the early stages of research on nano-building block synthesis, zero dimensional shapes were regarded as the most basic and symmetric, including spheres and cubes. Several semiconductor nanocrystals have been grown from the ageing processes of ionic precursors inside organic micelles. However, nanocrystals obtained by this method have relatively poor crystallinity or polydispersity in their size. As an alternative way to solve these problems, a thermal decomposition method of organometallic precursors under hot organic solution was adopted. At first, Chestnoy et al (1986)[7] synthesized various II–VI semiconductor nanospheres with high colloidal stability, using coordinating solvents (e.g., 4-Ethylpyridine), but the size tunability and monodispersity of the nanocrystals obtained, were still poor. Murray et al (1993)[8] successfully developed a more advanced methodology to prepare CdSe nanocrystals of varied sizes via the method of injecting a precursor solution containing dimethylcadmium and trioctylphosphine selenide into a hot trioctylphosphine oxide (TOPO) solution. The size of nanocrystals varied from 1.2 to 12 nm with high monodispersity and crystallinity; the nanocrystals obtained were highly soluble in various organic solvents. Optical spectra clearly exhibited size dependent quantum confinement effects, indicating the high monodispersity and high crystallinity of nanocrystals.
4.2. Quasi One Dimensional (1D) Nanostructures
- The term quasi one dimensional nanostructures is used, because the dimensions are often larger than the indicated threshold, although elongation along one main axis still exists. When the diameter of the nanorod, nanowire or nanotube becomes smaller, there is often a significant change in the properties with respect to crystalline solids or even two dimensional systems. A bismuth nanowire is an excellent example, which transforms into a semiconductor, as the wire diameter becomes smaller. By controlling the growth variables such as temperature, the choice of capping molecules, precursor concentrations, crystalline phases of the nuclei and the choice of the regime between kinetically controlled and thermodynamically controlled growth, various nano-building blocks with multi-dimensionalities have been produced. To generate one dimensional nanocrystals, researchers have explored the ‘one step in situ synthesis’ of 1D nanorods, utilizing methods similar to those for the well studied spherical nanocrystals. For example, the use of binary capping molecules such as TOPO and hexylphosphonic acid (HPA) was effective for the generation of shape anisotropy in CdSe along with the intrinsic hexagonal structure nature.The non-hydrolytic high temperature injection method can be effectively utilized, for high quality nanorods synthesis. Peng et al (2000)[9] and Manna et al (2000)[10] first reported CdSe nanorods via thermal decomposition of dimethylcadmium and trioctylphosphine selenide, in a hot surfactant mixture of trioctylphosphine oxide and hexylphosphonic acid. The hydrolytic synthesis of II–VI semiconductors also produces one-dimensional rod-shaped nanocrystals, by shape transformations involving oriented attachment processes. Tang et al (2002)[11] reported a shape transformation from a sphere to a rod by the dipole-induced fusion of CdTe individual nanospheres. III–V Semiconductor one-dimensional nanocrystals including InP, GaAs and InAs can also be synthesized via solution–liquid–solid (SLS) processes. In the case of group IV semiconductor systems, it is extremely difficult to obtain nanorods by typical solution based precursor injection methods, due to their highly covalent character. In contrast, Morales and Lieber (1998) used gas phase synthesis such as chemical vapor deposition, where one-dimensional silicon & germanium wires can be easily obtained on a substrate, using vapour–liquid–solid (VLS) growth mechanisms. Transition metal oxides consist of an important group of materials used in white pigment, electronic ceramics, cosmetics, support in catalysis, and as photocatalysts. Nanostructured titania are of particular interest, with potential applications as solar cell materials. Chemseddine and Moritz (1999) demonstrated elongated TiO2 nanocrystals synthesized by hydrolysis and polycondensation of titanium alkoxide[Ti(OR)4], in the presence of tetramethyl ammonium hydroxide, as a stabilizer and reaction catalyst. Penn and Banfield (1999) also reported naturally aligned titania nanocrystals under hydrothermal conditions, by adopting an oriented attachment mechanism into the nanocrystal development. The hydrothermal treatment of titanium alkoxide precursors produces diamond shaped anatase titania nanocrystals.
4.3. Two Dimensional (2D) Nanostructures
- The family of 2D nanosystems encompasses all those systems that exhibit two dimensions exceeding the third one. However, the number and variety of inorganic nano objects belonging to this family is far lower. with respect to 0D and 1D nanosystems. Indeed, nature tends to organize materials in a three dimensional way. 2D assemblies usually do not grow except under special and controlled experimental conditions. The main synthesis methodologies of 2D nanostructures can be summarized as follows: (i) anisotropic crystal growth, (ii) surfactant-assisted synthesis and (iii) the assembly of simpler 0D or 1D nanosystems.All 2D flat nanocrystals possess an overall size in the order of 10 nm. Such a size limitation is pursued, in order to prevent the growth along only one specific direction, leading to a 1D system. The synthesis of two dimensional nanocrystals is achieved by the self-assembly of solutions and the constituting elements of these systems are usually metals. Discoidal nanocrystals are typical flat building blocks. They are typically obtained by surfactant assisted synthesis, or anisotropic crystal growth passing through colloidal systems.2D prismatic shapes can be prepared by photoinduced shape changes. Silver nanoprisms are synthesized by the irradiation of Ag nanospheres with visible light together with an unexpected colour change (from yellow, which is a characteristic surface plasmon band of the spherical particle, to green and finally blue), and a marked change in shape, from nanospheres into nanoprisms[12]. Nanosized prisms from transition metals, such as Pd or Ni or from semiconductors, such as CdS, were also reported. The triangular nanocrystals of CdS turned out to be flat and the crystalline phase was proved to be a hexagonal wurtzite structure.
4.4. Three Dimensional (3D) Nanosystems
- Objects having either an overall size in the non-nanometric range (mainly in micrometer or millimeter range), but displaying nanometric features (such as nanosized confinement spaces) or resulting from the periodic arrangement and assembly of nanosized building blocks, can be classified as ‘3D nanosystems’. They exhibit different molecular and bulk properties. In particular, 3D nanocrystals superstructures are prepared by assembling basic nanosized building blocks such as; 0D spheres 1D rods and 2D plates, to have bigger sized structures of innovative shapes. On the contrary, nanoporous materials are made with a ‘complementary’ approach, since a system of nanosized void pores is obtained within a continuous bulk material. Simpler nanosystems can otherwise be used as ‘artificial atoms’ to build three-dimensional superstructures, such as superlattices in which a given nanoparticle is in a predictable and periodic lattice point. For this purpose, 0D nanosystems (and mainly nanoparticles) are the best choice, since they can easily lead to the highly ordered 3D closely packed patterns, kept together by chemical interparticle interactions. Superlattices of CdSe nanocrystals can be obtained, using a selective evaporation technique from a solution of octane & octanol containing spherical CdSe nanocrystals. Such superstructures display a face centered cubic packing of CdSe nanocrystals. They exhibit novel optical properties which are different from those of diluted CdSe nanospheres in solution.
5. Core-Shell Nanostructures
- Surface engineering is an important tool to control the properties of the NCs and in particular optical materials. One important strategy is the over growth of NCs with a shell of a second semiconductor, resulting in a core-shell (CS) system. This method has been successfully applied to improve the florescence quantum yield and the stability against photo-oxidation by the proper choice of the core and shell materials, to tune the emission wavelength in a large spectral window. After pioneering work in the 1980s, and the development of powerful chemical synthesis routes at the end of 1990s[13-15], a strongly increasing number of articles have been devoted to CSNCs in the last five years. Nowadays, almost any type of core NCs prepared by a robust chemical synthesis method has been over grown with shells of other semiconductor materials. Depending on the bandgap and the relative position of the electronic energy levels of the involved semiconductors, the shell can have different functions in core-shell nanocrystals (CSNCs).
5.1. Types of Core-shell Nanocrystals
- Two main cases are nominated as type I and type II band alignment, respectively, in which the type-I core shell has a band gap of the shell material larger than the core, and both the electrons and holes confined in the core. In the latter, either the valence band edge or the conduction band edge of the shell material is located in the band gap of the core. The resulting staggered band alignment leads on the excitation of the NC, to a special separation of the hole and the electron in the different regions of the CS structure. Type I heterostructures experience optical transition between the electron and hole states, whose wave functions are localized in the same region in real space, whereas for type II hetrostructures, the electron and hole lie in different regions (here, the core and shell of the NCs). In type I CSNCs, the shell is used to ‘passivate’ the surface of the core, with the goal to improve its optical properties. The shell separates physically the optically active surface core NC from its surrounding medium. Consequently, the sensitivity of the optical properties changes in the local environment of the NCs surface. With respect to the core NCs, the core-shell (CS) systems generally enhance the stability against photodegradation. At the same time, the shell growth reduces the number of surface dangling bonds, which can act as trap states for charge carriers and reduce the fluorescence quantum yield (QY). The first published prototype system was CdSe/ZnS[15], where the ZnS shell significantly increases the fluorescence QY and stability against photobleaching. Shell growth is accompanied by a small shift (5-10 nm) of the excitonic peak in the UV-Vis absorption spectrum and the PL wavelength. This is attributed to a partial leakage of the exciton in to the shell material. In type II systems, the shell growth aims at a significant red shift of the emission wavelength of the NCs. The staggered band alignment leads to a smaller effective band gap, than that of each one of the constituting core and shell materials. These systems tune the emission colour with the shell thickness towards spectral ranges, which are difficult with other materials. Type II CSNCs have been developed in particular for near IR emission, using CdTe/CdSe or CdSe/ZnTe. In contrast to the type I systems, the PL decay times are strongly prolonged in type II CSNCs, due to the lower overlap of the electron and hole wave functions. As one of the charge carriers (electron or hole) is located in the shell, an over growth of type II CSNCs with an outer shell of an appropriate material can be used in the same way as in type I systems, to improve the fluorescence QY and photostability.
6. Quantum Confinement Effects
- The quantum confinement effects in low dimensional semiconductor systems were studied two decades ago. In the last decade, comprehensive, well written reviews appeared which concentrated on the quantum confinement effects of various semiconductors with the emphasis on the optical properties, including absorption and luminescence[16,17]. Obviously, the confinement of an electron and hole in nanocrystals significantly depends on the material properties, namely, on the Bohr radius aB. These effects take place in bigger nanocrystals and depend on the material properties, namely, on the Bohr radius aB = 2.34 nm and aB of about 10 nm, which would have Cd related compounds such as CdTe, CdZnTe and CdTeSe. One of the most important consequences of the spatial confinement effect is an increase in the energy of the band -to-band excitation peaks (blue shift), as the radius R of a microcrystalline semiconductor is reduced in relation with the Bohr radius. Theoretically, the regimes of quantum confinement differ in their main electron-hole interaction energy, i.e., the Coulomb term and the confinement energy of the electron and hole and kinetic energy.
6.1. Weak Confinement Regime
- To observe this regime, the radius R of a crystallite should be greater than the bulk exciton Bohr radius aB. In this region of weak confinement, the dominant energy is the Coulomb term and there already occurs a size quantization of the exciton motion. The exciton energy states are shifted to higher energies by confinement and the shift in energy ∆E is proportional to 1/R2. The shift ‘∆E’ of the exciton ground state is given approximately by
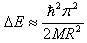 | (1) |
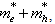 with
with 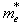 and
and 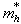 being the effective masses of the electron and hole respectively.
being the effective masses of the electron and hole respectively.6.2. Moderate Confinement Regime
- The moderate confinement regime occurs when R≈ aB and ah < R < ae, where, ah and ae are the hole and electron Bohr radii, respectively. In II-VI semiconductors, this region is well observable in small QDs. Its characteristic feature is the well restricted motion of a photoexcited hole.
6.3. Strong Confinement Regime
- Finally, the size of a QD can be decreased in such a way that R<< aB and R << ah and ae in the strong confinement regime. The Coulomb term of electron-hole interaction is now small and can be ignored or treated as perturbation. The electrons and holes can now be thought of as confinement independent particles. So excitons are not formed and the separate size quantization of an electron and hole is the dominant factor. The optical spectra consist also of a series of lines due to the transition between sub-bands. This factor was confirmed experimentally and the simple model gives the shift in energy as a function of crystallite size as,
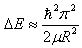 | (2) |
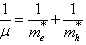 | (3) |
7. Nanoparticles Synthesis Methods
7.1. Wet Chemical Methods
- The drive for miniaturization with the corresponding demand for smaller machines and components using less resources and energy that occurred during the last two decades, have been rapidly pushing the industry to the atomic and nanometer scale. The development of new synthesis strategies for advanced materials with enhanced properties and affording an effective control at the nanometer level is therefore required. Amongst the available synthetic methods employed for the development of nanosized systems, there are two main general approaches: “bottom-up” and “top-down”. The former applies to the creation of organic and inorganic structures, atom-by-atom or molecule-by-molecule. It implies the use of atoms or molecules as building blocks, to design and assemble nano arrangements of atoms in a functional form to give macroscopic systems.The concept of ‘organic–inorganic hybrid’ materials emerged very recently with the birth of ‘soft’ inorganic chemistry processes, particularly the solvothermal / hydrothermal and sol–gel process which is probably the supreme soft inorganic chemistry process, allowing a tailored chemical design. The unique low temperature processing characteristics of this process allow the chemical design of organic–inorganic hybrid materials through the incorporation of low molecular weight organic molecules with appropriate functionalities into inorganic moieties, at temperatures at which the organic ones are not destroyed.It is in the design and characterization of advanced materials, that the importance of new interdisciplinary studies may be realized. Thus, for the design of novel advanced functional nanomaterials, the uniformity of the shape and size of the nanoparticles is a key issue. NC samples must be monodisperse in terms of size, shape, internal structure and surface chemistry. Nanoparticles are considered monodisperse when their size distribution is < 5%. In the past decade, a particular interest was taken in the development of new synthetic routes, enabling the rigorous control of the morphology and size of the nanoparticles. The preparation of nanoparticles can be achieved through different approaches, either chemical or physical, including gaseous, liquid and solid media. While physical methods generally tend to approach the synthesis of nanostructures by decreasing the size of the constituents of the bulk material (top-down approach), chemical methods tend to attempt to control the clustering of atoms/molecules at the nanoscale range (bottom-up approach) Figure 2. However, the wet chemical methods are the most popular, because they present several major advantages over the other conventional methods, i.e., they are highly reliable and cost effective, allow a much more rigorous control of the shape and size of the nanoparticles and the agglomeration of the resulting particles can be alleviated by functionalization with different capping ligands[18]. The wet chemical synthesis routes offer an easy way to yield colloidal solutions with a large range of particle sizes on the gram scale in combination with high reproducibility. The following wet chemical methods have been successfully employed for developing different types of nanoparticles:1. Sol-Gel2. Solvothermal/hydrothermal3. Co-precipitation
7.2. Sol-Gel
- The term sol gel was first coined in the late 1800s. It generally refers to a low temperature method using inorganic precursors, that can produce ceramics and glasses with better purity and homogeneity than through high temperature conventional processes[19]. Two most attractive features of the sol gel process are that, it can produce compositions that cannot be created with conventional methods and that the mixing level of the solution is retained in the final product, often up to the molecular scale. Because of the potential in the fabrication of a wide variety of new materials, an understanding of sol-gel processes has become the centre of interdisciplinary research, viz., physics, chemistry, biology, biotechnology, bio chemistry, electronics and related engineering branches. Sol-gel materials have a wide range of applications such as environmental protection, solar cells, energy storage, ceramics, sensors, magnetic devices, etc. The main advantage of the sol gel technology is the possibility to control the mechanism and kinetics of the proceeding chemical reactions, in other words, controlling each step of the sol gel processes. A single step may affect the final structure of the materials and cause the modification of the process[20].
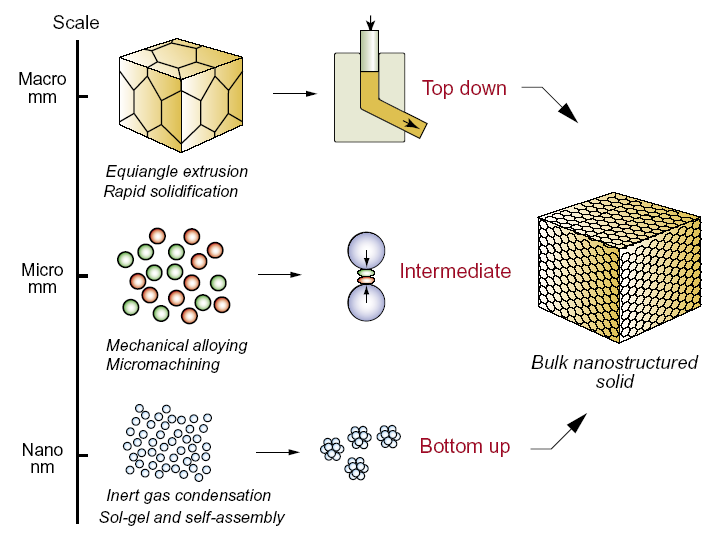 | Figure 2. The top-down, intermediate and bottom-up approaches to making bulk nanostructured solids |
7.3. Solvothermal/Hydrothermal Method
- The solvothermal route of synthesizing materials is a relative newcomer to the toolkit for materials scientists and chemists. Twenty years after the first development of solvothermal reactions, it appears important through the last research activities to trace future trends, taking into account their potentialities and the different economical constraints. Solvothermal reactions have been mainly used for preparing micro or nanoparticles with different morphologies. Due to the necessity of developing new materials for basic research or industrial applications, the recent interest has been focused on the potentialities of solvothermal reactions in materials synthesis. The solvothermal technique finds an increasing use in nanotechnology and offers a unique means of making highly functionalized materials, for applications such as sensors, separation and catalysis, molecular devices and spintronics[21]. The solvothermal process can be defined as “A chemical reaction in a closed system in the presence of a solvent (aqueous and non-aqueous solution) at a temperature higher than that of the boiling point of such a solvent”. Consequently, a solvothermal process involves high pressures. The selected temperature (sub or supercritical domains) depends on the required reaction for obtaining the target material through the involved process. In the case of an aqueous solution as a solvent, hydrothermal technology has been studied and developed a long time ago with different objectives such as; 1. Mineral extraction (as for leaching ores )[22] 2. Investigation of the synthesis of geological materials [23] 3. Synthesis of novel materials[24-26] 4. The deposition of a thin film[27] 5. The development of the sintering process in mild conditions 6. The elaboration of fine particles, well defined in size and morphology[28] In particular, the hydrothermal process of the chemical composition of water as the solvent is mainly appropriate to the preparation of hydroxides, oxihydroxide or oxides versus the temperature value. The development of non-oxide metals (in particular nitrides, calcoginides etc.) for investigating their physical properties and industrial applications requires the development of a new process involving non-aqueous solvents. Consequently, if solvothermal reaction is a “generic term” for a chemical reaction in a closed system in the presence of a solvent, these reactions are mainly developed with non-aqueous solvents for preparing non-oxide materials.
7.3.1. Main Parameters Governing Solvothermal Reactions
- Generally, two parameters are involved in solvothermal reactions: a. Chemical and b. Thermodynamical
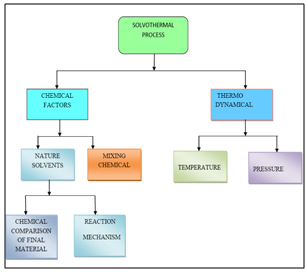 | Figure 3. Main factors governing solvothermal processes |
7.4. Surface Modification of Nanocrystals and Interparticle Forces in Solution
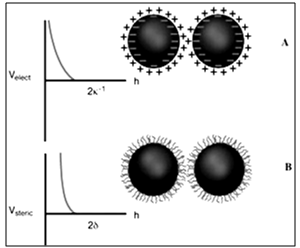
- To produce well-defined 2D and 3D superlattices of nanocrystals, highly stable materials are needed. Furthermore, various forces have to be taken into account. Let us first list the various parameters involved in nanocrystal self-assemblies. Due to Van der Waals interactions, particles in the nanometer-size range have a strong tendency to agglomerate (Figure 4). It is therefore important to develop synthetic methods by which the particles can be stabilized, i. e., where repulsive and attractive forces between particles balance each other mainly electrostatic and steric forces that prevent agglomeration of nanoparticles. Electrostatic stabilization involves the creation of an electrical double layer arising from ions adsorbed on the surface and the associated counter ions that surround the particle. Thus, if the electric potential associated with the double layer is sufficiently high, the Coulomb repulsions between the particles prevent their agglomeration (Figure 5). Steric stabilization is achieved by the adsorption of organic molecules containing suitable functional groups, such as –SH, –COOH and –NH2, at the particle surface. Indeed, the lengths of the alkyl chains are usually greater than the range over which the forces of attraction between nanocrystals are active. In addition, dipolar magnetic interactions are to be taken into account for single-domain magnetic nanocrystals. Hence, the stability of a colloidal solution is governed by the total interparticle potential energy Utotal, which can be expressed as:
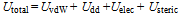 | (4) |
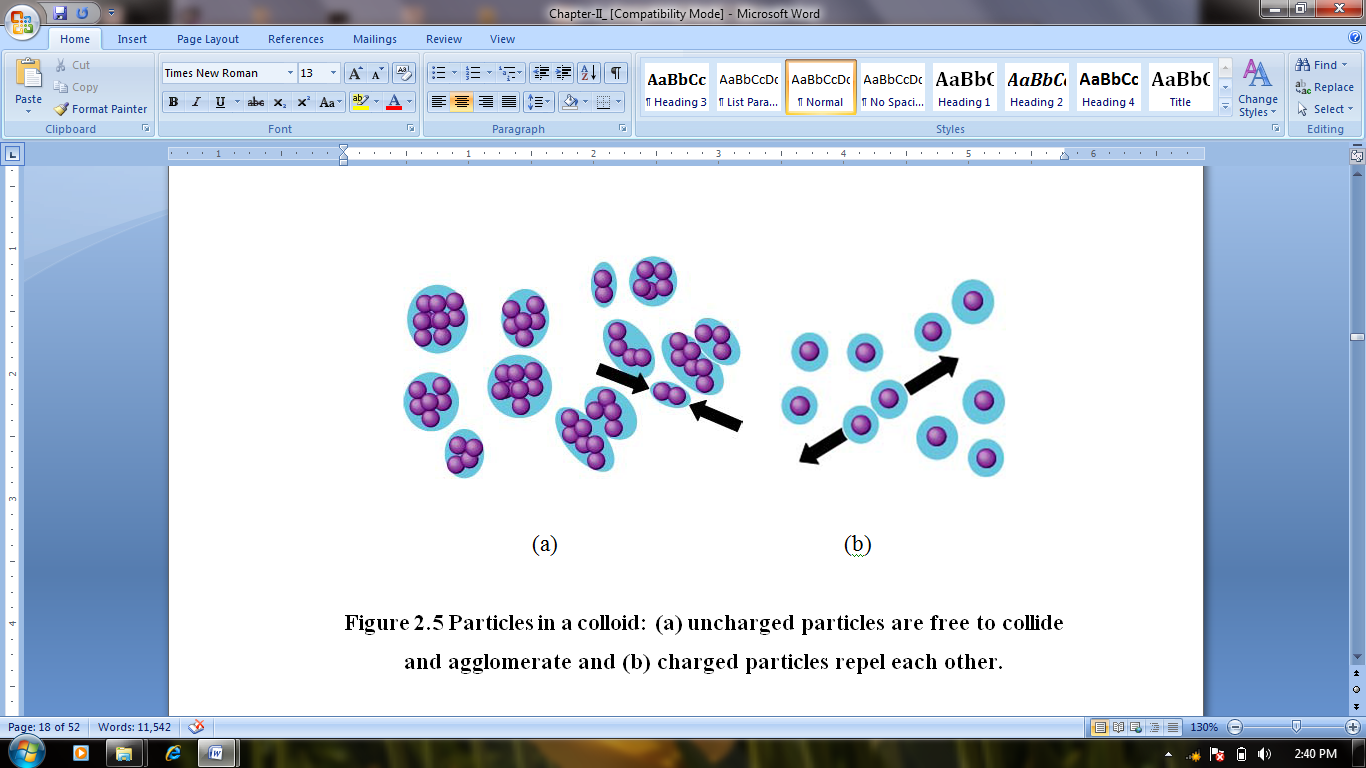 | Figure 4. Uncharged particles are free to collide and agglomerate |
7.5. Van der Waals Forces
- UvdW exhibits a power-law distance dependence, whose strength varies with the Hamaker constant and a geometrical factor[31, 32]. The Hamaker constant (Ap–o–p) depends on the dielectric properties of the interacting colloidal particles (p) and intervening solvent (oil) and it is higher for metallic materials than for semiconductors. The geometrical factor depends on the particle size and the contact distance between the nanocrystals. For spherical particles (i, j) of equal size, UvdW is given by the Hamaker expression:
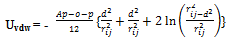 | (5) |
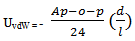 | (6) |
7.6. Magnetic Dipolar Forces
- The magnetic dipolar energy between spherical nanocrystals of equal size and with the same magnetic properties is expressed as[33]
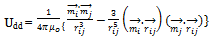 | (7) |
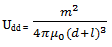 | (8) |
7.7. Electrostatic Forces
- The electrostatic potential energy between charged particles, Uelec exhibits an exponential distance dependence, whose strength varies with the surface potential induced on the interacting colloidal particles and with the dielectric properties of the intervening medium[35]. Exact analytical expressions for the electrostatic potential energy cannot be given. Therefore, analytical approximations or numerical solutions are used. For equal-sized spherical particles that approach one another under constant potential conditions, Uelec is given by:
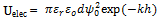 | (9) |
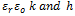 are the dielectric constant of the solvent, the permittivity of vacuum, the surface potential, the minimum separation distance between particles and the Debye–Hückel screening length, respectively; k is given by;
are the dielectric constant of the solvent, the permittivity of vacuum, the surface potential, the minimum separation distance between particles and the Debye–Hückel screening length, respectively; k is given by;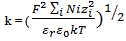 | (10) |
7.8. Steric Forces
- Steric stabilization provides an alternative route to control the colloidal stability and is used in aqueous and nonaqueous solutions. In this approach, the adsorbed organic molecules are utilized to induce steric repulsion. To be effective, the adsorbed layers must be of sufficient thickness and density to overcome the Van der Waals attraction between particles and to prevent bridging flocculation. Such species should be strongly anchored to avoid desorption during particle collisions. Steric interactions occur when particles approach one another at a separation distance less than twice the adlayer thickness (δ). Usteric is given by[37]
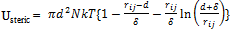 | (11) |
7.9. Solvation Forces
- The use of appropriate solvents is required for high particle stabilization. The stabilizing agent has to possess high affinity with the solvent in order to solvate the particles and form an extended layer for screening the attraction between the particles[38].
8. Application of Semiconductor Nanomaterials
- Semiconductor nanomaterials have interesting physical and chemical properties and useful functionalities, when compared with their conventional bulk counterparts and molecular materials. Narrow and intensive emission spectra, continuous absorption bands, high chemical and photobleaching stability, processability, and surface functionality are among the most attractive properties of these materials. The development of “nanochemistry” is reflected in an immense number of publications on the synthesis of semiconductor nanoparticles[39]. For instance, the spatial quantum confinement effect results in significant change in optical properties of semiconductor nanomaterials. The very high dispersity (high surface-to-volume ratio), with both physical and chemical properties of the semiconductor has a major influence on their optical and surface properties. As a result, semiconductor nanomaterials have been the focus of research for about 20 years and have attracted significant interest in research and applications in diverse disciplines such as solid-state physics, inorganic chemistry, physical chemistry, colloid chemistry, materials science, and recently biological sciences, medical sciences, engineering, and interdisciplinary fields. Among the unique properties of nanomaterials, the movement of electrons and holes in semiconductor nanomaterials is primarily governed by the well-known quantum confinement, and the transport properties related to phonons and photons are largely affected by the size and geometry of the materials[40-43]. The specific surface area and surface-to-volume ratio increase drastically as the size of the material decreases[40, 44]. Parameters such as size, shape, and surface characteristics can be varied to control their properties for different applications of interest[45]. These novel properties of semiconductor nanomaterials have attracted significant attention in research and applications in emerging technologies such as nanoelectronics, nanophotonics, energy conversion, non-linear optics, miniaturized sensors and imaging devices, solar cells, catalysis, detectors, photography biomedicine etc., In this section we discuss the application of semiconductor nanomaterials in catalysis and medical sciences.
9. Semiconductor Nanomaterials for Hydrogen Production
- The extensive use of fossil fuel over the last 150 years has caused a rise in urban ill-health, economic dependence, political unrest and many cases of warfare[46]. A recent European study revealed that more deaths are caused by car emissions than by car accidents[47], and a parallel Swedish study reported that pollution can increase the risk of having cancer by 40-70 %[48] and recent atmospheric investigations have also shown a substantial increase in the concentrations of carbon oxide and other greenhouse gases, with potentially threatening consequences to the global climate. Growing environmental concerns are related to the extensive use of non-sustainable fossil fuels (oil, natural gas and coal) and a constantly increasing energy demand for clean and sustainable sources of energy. Hydrogen is a promising alternative fuel, since it is completely pollution-free and can readily be produced from renewable energy resources, thus eliminating the net production of greenhouse gases. Recent studies have indicated that hydrogen fuel costs are reasonable and hydrogen is therefore an ideal candidate to replace fossil fuels as an energy carrier. The production of hydrogen from liquid hydrocarbon fuels and adsorption on various solids including carbon has attracted a lot of attention by the media and the automotive industry. There is increasing environmental pollution, caused by combustion engines, and additional problems associated with large-scale mining, transportation, processing and usage of fossil fuel. Photocatalysis is expected to make a great contribution to both environmental treatment (emission cleaning and water purification) and renewable energy. Hydrogen (H2) is widely considered to be the future clean energy carrier in many applications, such as environmentally friendly vehicles, domestic heating, and stationary power generation. Photocatalytic hydrogen production from water in one of the promising techniques due to the following advantages:ØIt is based on photon (or solar) energy, which is a clean, perpetual source of energy, and mainly water, which is a renewable resource.Ø It is an environmentally safe technology without undesirable by-products and pollutants. Ø The photochemical conversion of solar energy into a storable form of energy, i.e. hydrogen, allows one to deal with the intermittent character and seasonal variation of the solar influx. However, it requires a photocatalyst that should possess chemical stability, corrosion resistance, visible light harvesting and suitable band edges. The structural and electronic properties of semiconductor photocatalysts largely determine the process of photocatalytic H2 production, including basic steps such as the absorption of photons, charge separation and migration, and surface reactions. A semiconductor consists of a valence band (VB) and a conduction band (CB), which are separated from one another by a bandgap (Eg). The photon of energy hυ equal or greater than the bandgap energy (Eg) of the semiconductor particles produces an electron, e-cb, in the conduction band (CB) leaving a hole, h+vb in the valence band (VB). In the ground state, all electrons exist in the VB. Under irradiation by photons with energy equivalent to or larger than Eg, some of the electrons are excited from the VB to the CB, leaving empty states, called holes, in the VB. These photogenerated electrons and holes may recombine in the bulk or on the surface of the semiconductor on a time scale, which is slower than the time required for their formation. Electrons and holes that travel to the surface of the semiconductor before they recombine can cause reduction (H2 formation) and oxidation (O2 formation) reactions, respectively. For H2 production to occur, the CB bottom-edge must be more negative than the reduction potential of H+ to H2 (EH+/H2=0 V vs NHE at pH=0), while the VB top-edge should be more positive than the oxidation potential of H2O to O2 (EO2/H2O=1.23 V vs NHE at pH=0) for O2 formation from water to occur. The whole process is given in Figure 6.Semiconductors such as TiO2, ZnO, ZrO2, V2O5, WO3, Fe2O3, SnO2, CdSe, GaAs, GaP and metal sulphides (CdS and ZnS) are employed as photocatalysts. Among the semiconductors, titanium dioxide (TiO2) is one of the most important and widely used photocatalysts, because of its suitable flat band potential, high chemical stability, nontoxicity, corrosion resistance, abundance, cheapness, and high photocatalytic activity. TiO2 is used not only for the photocatalytic degradation of environmental pollutants; it also applied to produce and storage of hydrogen gas. The use of semiconductosr other than TiO2 such as ZnO, ZrO2, V2O5, WO3, Fe2O3, SnO2, CdSe, GaAs, GaP, CdS and ZnS have the drawback of instability and/or corrode easily, either dissolving or forming a thin film, which prevents the electrons from transferring across the semiconductor/liquid interface. The morphology of TiO2 plays a very important role in the efficiency of photocatalysis for H2 production. One-dimensional TiO2 (nanowires, nanorods, nanotubes and nanofibers) has attracted more and more attention. Compared to spherical particles, one-dimensional TiO2 nanostructures could provide a high surface area and a high interfacial charge transfer rate. In particular, the particle size, chemical composition (including dopants), microstructure, the crystal phase, morphology, surface modification, bandgap and flat-band potential of the nanophotocatalysts have shown a visible effect on photocatalytic H2 production rates, which may be further increased by adding sensitizers, cocatalysts or scavengers. The biggest disadvantage of TiO2 is that it is inactive under visible light irradiation, due to its large bandgap, which impedes the use of TiO2 as a solar energy harvesting photocatalyst. In order to overcome these deficiencies, TiO2 doped with different metals, nonmetals, and surface modifications has been used to utilize solar energy as the irradiation source.Nanocomposites have been studied extensively in photocatalytic H2 production. The intercalation of semiconductor nanoparticles such as TiO2, CdS, Cd0.8Zn0.2S and Fe2O3 into layered compounds such as H2Ti4O9, H4Nb6O17, K2Ti3.9Nb0.1O9, HNbWO6, HTaWO6, HTiNbO5 and HTiTaO5 has been reported for H2 production[49-52]. The intercalation suppresses the growth of the nanoparticles. When these intercalated nanoparticles are excited by bandgap irradiation, the photogenerated electrons can be quickly transferred to the matrix layered compounds. For example, in the LaMnO3/CdS nanocomposite, the holes photogenerated by visible light in the valence band of CdS can move to the valence band of LaMnO3 and react with electron donors (Na2S and Na2SO3), while the photogenerated electrons remain in the conduction band of CdS and react with water to produce H2. This charge–carrier separation at the nanoscale is responsible for the improved photocatalytic activity[53]. Photocatalytic H2 production offers unique opportunities to develop an alternative and sustainable energy system and to reduce the emission of greenhouse gases. Photocatalyst materials play a crucial role in photocatalytic H2 production. Nanostructured photocatalysts are expected to be a future trend, since nanosized photocatalysts have shown much better performance than their bulk counterparts. Many of semiconductor nanomaterials have been used to produce H2. Theoretical and modelling work is useful and imperative for a better understanding of the process and mechanism of nanophotocatalytic H2 production as well as for designing new nanophotocatalysts and miniphotoreactors. The charge and mass transport processes in these areas, which are predominant in nanostructures, are different from those in the bulk phase. In most cases, photocatalytic H2 production can be increased drastically by modifying the catalyst's nanostructure with the cocatalysts. In order to enhance the photocatalytic efficiency of semi-conductors, Serpone et al. proposed an interparticle electron transfer process, by coupling two semi-conductors with different redox energy levels to increase the charge separation for the corresponding conduction and valence bands and absorb visible light. The semiconductor photocatalyst TiO2 and ZnO were coupled with another semiconductor nanomaterial such as CdS[54], SnO2[55], WO3[56], Bi2S3[57], Cu2O, Bi2O3, Fe2O3[58], CdSe[59], ZrO2[60], ZnMn2O4[61], and In2O3[62]. For example, the TiO2 nanotube was coupled with CdS semiconductor nanomaterials by the ion-exchange and precipitation method[63].
10. Silicon Semiconductor Nanomaterials and Devices
- This field has become one of the most active research areas of semiconductor nanotechnology. Knowledge of modern information technology is based on silicon based microelectronics. Therefore, the development of silicon based materials and devices will influence the future development of current microelectronics and information technology to a certain extent, which has an important strategic significance. Besides the widely used crystalline silicon wafers, silicon nanostructures have been considered as the basic components for future nano/microelectronic devices. With the size of silicon based complementary metal oxide semiconductors (CMOS) circuit reducing gradually down to 10 nm or even smaller, a series of severed challenges appear, such as how to deal with device manufacturing limits, dramatic increase in cost, and the working mechanism changes of new devices. These lead to the ‘bottle-neck’ of the future development in the silicon based microelectronics industry, while silicon based nanotechnology can provide a solution with lower cost and higher efficiency. The size effect of silicon nanostructures leads to a lot of novel properties. One typical example is the size-tunable highly-luminescent silicon nanostructures due to the quantum effect. By using these novel properties scientists have recently developed several silicon based novel devices like highly sensitive biological and chemical sensors, high-efficiency solar cells and light-emitting diodes. Therefore, silicon nanostructures show wide application prospects in many fields. Our aim is to build an internationally advanced scientific center for nanotechnologically research and to create a competitive technology innovative environment for nanotechnology applications.
11. Research on Nano Optoelectronic Sensors and Photovoltaic Devices
- This area includes surface modification, aligned assembly of nanomaterials, and conjugation of nanomaterials with bio-molecules. Employing the interactions between nanomaterials and analytes, and the trace detection of target samples based on the property change in optics, electronics, magnetics, sound, force, and their combinations, nano chemical sensors will be very useful in the applications of safety, health, hygiene, and anti-terrorism. Nanosized semiconductor optoelectronic sensors and photovoltaic devices have several advantages like energy saving, high efficiency, and high stability, which make them more and more useful in many applications.
12. Organic Optoelectronic Materials and Devices
- Much research has been focused on a series of highly functional organic molecules, such as organic optoelectronic materials with strong photoluminescence, nonlinear optical materials, and some important low molecular weight pharmaceutical materials, etc. First, is the design and synthesis of organic compounds with intended functions. Second, is to controllably prepare the corresponding nanomaterials through various pathways, followed by structure characterization and theoretical calculation/simulation, to analyze the relationship between the lattice structure and nanostructure. Third, by investigating the differences between organic nanomaterials and bulk or molecular state materials in their optical, electronic, catalytic, chemical and sensor-related properties, try to discover the advantages of organic nanomaterials, construct the nanodevices and explore their practical applications. Organic optoelectronics has developed into a new interdisciplinary research field, involving organic chemistry, physics, electronic engineering and materials science. Organic optoelectronic devices, such as organic electroluminescent devices (OLED), organic photovoltaic (OPV) and organic thin film transistors (OTFT), have attracted significant attention in academics and industries, due to their great application potential in flat-panel and flexible display, solid-state lighting, information transport and storage, new generation energy, photocatalyst and so on. Owing to the advantages of solid-state, self-emission, full color capability and flexibility, OLED has been recognized as one of the most promising flat-panel display technology and has just become into commercial.
13. Conclusions
- Semiconductor nanomaterials are advanced materials for various applications, which have been discussed at length. The unique physical and chemical properties of semiconductor nanomaterial make it suitable for application in emerging technologies, such as nanoelectronics, nanophotonics, energy conversion, non-linear optics, miniaturized sensors and imaging devices, solar cells, detectors, photography and biomedicine. There are three key steps in the development of nanoscience and nanotechnology: materials preparation, property characterization and devices fabrication. The preparation of nanomaterials is being advanced by numerous physical and chemical techniques. The purification and size selected techniques developed can produce nanocrystals with well-defined structure and morphology.
ACKNOWLEDGEMENTS
- The author thank the Management and Principal of Sree Sastha Institute of Engineering and Technology, Chembarambakkam, Chennai-600123 for their encouragements throughout the work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML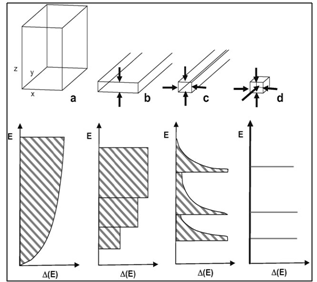
 Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites’, J. Phys. Chem. B, Vol.101, pp 9463-9475.
Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites’, J. Phys. Chem. B, Vol.101, pp 9463-9475.