-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2013; 3(3): 56-61
doi:10.5923/j.nn.20130303.05
Parametric Study of Silver Nanoparticles Production Using Submerged Arc-discharge Technique in De-ionized Water
1Consultant to Nanotechnology for Housing & Building National Research Center (HBRC), Dokki, Cairo, Egypt
2Instructor in Al-Ahram Higher Institute for Engineering and Technology, 6th October City, Giza, Egypt
Correspondence to: Mohamed A. Etman, Consultant to Nanotechnology for Housing & Building National Research Center (HBRC), Dokki, Cairo, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Nanotechnology has become extremely important in enhancing the potential of many applications. Silver Nanoparticles (SNPs) have recently become the most promising functional materials in modern science. Fabrication and characterization of silver Nanoparticles has attracted considerable attention as a result of their significant applications in Nano-technology and Nano-biotechnology. The unique physical, chemical and mechanical properties of Nanoparticles are the effect, among other things, of the high ratio of total surface area to their volume. The purpose of this paper is to conduct a parametric study to investigate the effect of relevant different parameters that affect the SNPs yield rate, structure and quality during their production in lab, using the submerged DC arc in de-ionized water technique. The set-up constructed specially for this purpose has been described. The effect of the current potential and intensity, the purity of the silver rods source and the combination of the electrodes sizes on the yield rate of SNPs has been studied. The produced silver nanoparticles have then been investigated using high resolution transmission electron microscopy (HRTEM) image and size distribution.
Keywords: Nanotechnology, Synthesis of Nanosilver, Submerged Arc Plasma Technique, HRTEM, Water Sterilizing Materials, Thermal Analysis
Cite this paper: Mohamed A. Etman, Parametric Study of Silver Nanoparticles Production Using Submerged Arc-discharge Technique in De-ionized Water, Nanoscience and Nanotechnology, Vol. 3 No. 3, 2013, pp. 56-61. doi: 10.5923/j.nn.20130303.05.
Article Outline
1. Introduction
- Metal nanoparticles have often been studied and used in nanotechnology. Silver has been used to preserve drinking water from germs since the periods of the Egyptian and Roman empires. It even has been used to preserve milk by putting silver coins in milk since the 18th century by immigrants to America. 1% of silver nitrate solution has been employed as eye drops to prevent eye disease in newborn since 1884[1]. In addition, silver foil has also been used to protect wounds from infection since World War I. To preserve the purity of drinking water on a spacecraft, since the 1970s, the National Aeronautics and Space Administration (NASA) have employed containers made from silver for preserving drinking water. However, since the human being overused too many antibiotics over a long time, thus some of bacteria, such as MRSA (Methicillin Resistant Staphylococcus Aureus)[2] and VRSA (Vancomycin Resistant Staphylococcus Aureus)[3] have become resistant to antibiotics. To overcome this problem, the physical and chemical properties of silver have been modified by raising the surface-to-volume ratio[4] of metallic Ag nanoparticles[5], which play an increasingly important role in the battle against germs.A thousand years ago silver and silver furniture were used for sterilizing and disinfecting purpose in the courts of the kings. Because of expensiveness, valuable properties of silver were not outspread used. Nanotechnology comes out into society reduce consumption of silver quantity in use while its properties were not only preserved but in most cases increased" (Sondi, 2003; Tien et al., 2008; Juehne, 2005). According to previous studies, very small quantity of 10 to 20 mg nanosilver metal per a cubic meter of water or about 200 mg per kilogram plastic material can avoid all acterial infects. Those affairs were explained by nanodimensional effects. Metal nanoparticles have become an important target for modern chemistry, physics, technology and bio-engineering. Fabrication and characterization of silver nanoparticles has attracted considerable attention as a result of their significant applications in the fundamental sciences and nanotechnology. The high surface-area-to volume ratio of nanoparticles can create their unique physical, chemical, mechanical, and quantum size effect properties[6]. Their potential applications include catalysis[7], photographic processes[8], and nanodetection by using surface-enhanced Ramam Scattering (SERS)[9], high-quality electrostatic precipitators, nanophotocatalysts in cosmetics, food, clothes, medicine, electrodes for MLCC (Multi Layer Ceramic Capacitors), silver fibers and others. Silver is a well-known bacteriostatic and poisonous agent for different bacteria and viruses[10]. No side effects were observed when using drugs based on metallic nano-silver in clinical trials. In principle, current techniques for nanometersized metal particle preparation can be divided into two categories: physical and chemical. UV and IR radiation[11], aerosol technology and lithography, evaporation[12] or laser ablation[13] from metal bulk samples are used to generate nanoparticles by physical methods. Reduction of metal ions into neutral metal clusters is a commonly used treatment in chemical synthesis. This includes conventional chemical (one or two phase system), photochemical[14], sonochemical[15], electrochemical [16], and radiolytic reduction[17]. A new method in synthesizing Ag nanoparticle suspension (SNPS), the arc discharge method (ADM)[18–21], has been successfully developed. From the experimental results and analysis, it was confirmed that this method was fast, simple, and easily adaptable to mass production. The synthesized SNPS contained the ionic Ag components which increase its germ-killing ability[22] and is capable of becoming a major weapon against germs in the postantibiotic era[23].The present paper describes a novel physical methodology of silver water suspension preparation by the arc-discharge method. Chemical methods for metal nanoparticle fabrication usually involve toxic chemicals, which can be dangerous to our environment. Although these methods may successfully produce pure silver nanoparticles, they require the use of stabilizers to protect the Ag nanoparticles against agglomeration. Additionally, these methods are usually expensive and potentially dangerous for the environment. Our studies have revealed that the DC arc-discharge between silver electrodes in pure water is a good alternative method, and is not only a relatively cheap process, but also environmentally friendly. As far as we know, this method of Ag nanoparticles preparation by arc-discharge in pure water has not been published in scientific literature. During arc-discharge the temperature between electrodes can reach several thousand °C[24] and the Ag rods used as electrodes are etched in the water medium. The vaporized metal can be condensed more efficiently in the dielectric liquid than in the gas phase. Silver vapor condensed in water creates a stable Ag aqueous suspension. Well separated nano-sized Ag clusters in pure water seem to be thermodynamically stable for a long time.
2. Experimental
- Silver wires purity up to(99.99%) have 1 mm in diameter and submerged in deionized water (pH = 6) were used as electrodes. The arc-discharge method is initially used for producing nanosilver. It is the most common and perhaps the easiest way to produce Silver nanoparticles (SNPs). However, it is a technique that produces a complex mixture of components and requires further purification to separate the SNPs from the soot metals present in the crude product. This method creates SNPs through arc vaporization of silver electrodes, separated by approximately 1 mm gap between two electrodes, in an enclosure filled with inert media.
2.1. Set-up
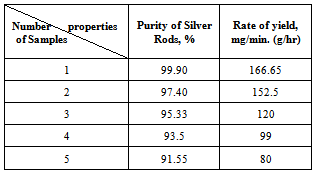
- In the present work a simple method for producing high-quality silver nanoparticles in relatively large quantities without the need of using expensive vacuum system equipment is developed. The set up construction was used to produced silver nanoparticles clarify in Figure 1 (a, b) and this technique proved to be economical, environmentally clean and produces uncontaminated nanoparticles". The main parts of set up are: i) two silver electrodes 1 mm in diameter, ii) a servo control system which maintains a constant distance between the electrodes, iii) a power supply system which controls the DC arc-discharge, iv) a glass container with an electrode holder and deionized water to collect the silver colloids, v) a stirring system with magnetic stirrer and stirring bar.
 | Figure 1. Set-up of Silver Nanoparticles production device |
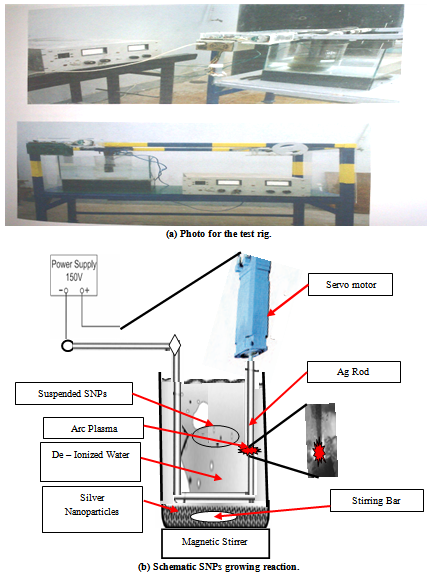
 | Figure 2. The plasma representation and photograph |
2.2. Experimental Procedures
- The arc-discharge was triggered between the pure silver electrodes while they were immersed to a depth of 3 cm in deionized water and the gap in between was maintained fixed at 1 mm. A current intensity up to 15 A through potential of 24 V can be achieved. The arc-discharge and the resulted plasma were found stable as long as the cathode-anode gap was maintained fixed. The discharge in this case can be designated as anodic arc-discharge. The direction of plasma expansion towards the anode is shown schematically in Figure 2(a). The bright illuminating zone that appears between the two silver electrodes shown in Figure 2(b) indicating to the potential difference between them due to applied arc plasma discharge to erosion of anode and precipitates into cathode.The plasma in this method is generated by the thermal evaporation of the anode material and thus the silver vapour is said to be generated by thermionic rather than thermo field emission. The produced SNPs by this technique can be categorized into two types: Silver nanoparticles that float on the water surface and the sediment products were precipitates in the glass container. The first floating type was found to be formed at maximum rate of 3 mg/min, while the later type, under the same experimental conditions, was found to be formed at 10 mg/min at most. This technique proved to produce economically high yield pure silver nanoparticles (SNPs) without the need for post microfiltration process. A set up of experiment has been conducted in order to investigate the factors affecting the synthesis of silver nanoparticles such as: the purity of the raw material of silver electrodes, the current intensity. Within the experimental limitations, optimum magnitudes of parameters have been determined.
2.3. Purification of Silver Nanoparticles (SNPs)
- A centrifugal separation device designated (Hermle Z-230), has then been found sufficient to concentrate the produced SNPs. The purification was carried out by successive separation and decantation using the centrifugal effect on four steps. Eventually, the suspended silver nano particles left over in water were found to be pure enough and no further separation process was needed.
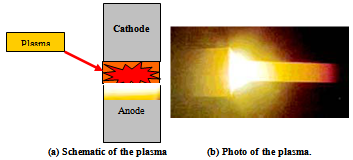
 | Figure 5. HRTEM images illustrate the SNPs growth (a) SNPs at scale bar 50nm, (b) transition stage of several SNPs at scale bar 10nm and (c) SNPs yield at high magnification with scale bar 5nm |
3. Silver Nanoparticles Characterization
- Carbon nanotubes are characterized and morphologically inspected using high resolution Transmission Electron (HRTEM), and Transmission Electron Diffraction (TEDM) Microscopy.
3.1. High Resolution Transmission Electron Microscopy (HRTEM)
- The HRTEM microscopy is used for studying the morphology of silver nanoparticles (SNPs) in time intervals of plasma discharge. The results are presented in the following sections.Samples of silver nanoparticles (SNPs) are sonicated in de-ionized water about 30 min and then put on a copper grid and allowed to dry slowly. Fig.5 (a, b and c) illustrates the high resolution transmission electron microscopy (HRTEM) of purified and dried samples of silver nanoparticles (SNPs) and HRTEM observe the size different between 1nm up to 3nm. Also, the lattices of silver nanoparticles (SNPs) products like network shape with small pore size marked by red circle are shown in Figure 5b.
3.2. Transmission Electron Diffraction Microscopy (TEDM)
- The diffraction pattern of TEDM image is used to identify the sizes of silver nanoparticles (SNPs). The sizes is determined from the equatorial oscillation is determined by measuring the distance from the different lines to the equatorial line. While it is possible to obtain the diffraction puller of the silver nanoparticles images, it is not possible to obtain the diffraction lines. The corresponding TEDM pattern of silver particles is illustration in Figure 6. When the electron diffraction is carried out on a limited number of crystals one observes only some spots of diffraction distributed on concentric circles. The rings patterns with different plane distances are consistent with the plane families of pure face-centred cubic (fcc) silver structure.
 | Figure 6. TEDM electron diffraction of SNPs |
4. Parameters Affecting Synthesis of Silver Nanoparticles
- In the arc-discharge technique adopted in the present experimental work, the structure and the yield rate of synthesized silver nanoparticles depend on different parameters such as:1. Current intensity and potential2. Purity of the electrodes silver material
4.1. Influence of Current Intensity on the Yield Rate of SNPs
- Figure 3 shows results of four groups of tests, in each group the current intensity covered values of 5, 10, 15, 25 amperes for certain combination of anode and cathode diameters. It can be observed that the highest obtainable DC current (15 A) resulted in highest yield rate of silver nanoparticles. In addition, the smallest used anode diameter of 1 mm together with the cathode diameter of 1 mm, produced at 15 amperes highest SNPs yield rate of 10 g/hr. Also, current intensity (15 A) has been found to produce relatively strong plasma tending to high rate of anode consumption and in turn resulting in high yield of SNPs. It is worth mentioning here that throughout these tests, purity of silver electrodes has been restricted at 99.9.
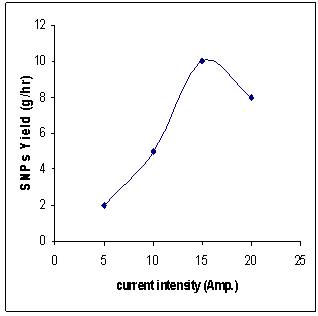 | Figure 3. Current intensity versus rate of yield of SNPs |
4.2. Effect of the Purity of Silver Electrodes on the Yield Rate of SNPs
- In order to investigate the effect of purity of the silver on the yield of SNPs, the five locally available types of silver electrodes have been tested for purity using the thermo gravimetric analysis method are shown in Table 1. The results have been found equal 99.9%, 97.4%, 95.33 %, 93.5% and 91.55% are given in Table 2. The results of the tests conducted on the test electrodes of different purities as related to both the yield rate of SNPs and the anode rate of consumption are plotted in Figure 4. It can be observed as expected that the highest available pure silver sample resulted in producing the highest yield of silver nanoparticles of 166.65 mg/min (10 g/hr) at DC current intensity of 15 amperes and consequently the highest rate of anode consumption as well. The rate of anode consumption helps in assessing the gap compensation required to keep the required plasma generation stable during the experimentation.It is worth mentioning here that throughout this part of experimentation the DC current is maintained fixed at 15A and the sizes combination of anode and cathode are chosen to be 1 mm.
|
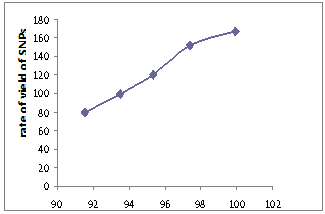 | Figure 4. Rate Yield of Silver Nanoparticles (SNPs) versus Silver Electrode Purity |
5. Conclusions
- In this paper the submerged arc in de-ionized water technique adopted to synthesize SNPs in lab and the test rig built specially for this purpose have been described. Experimental program has been designed and executed in order to investigate the effect of different parameters such as the arc current intensity, the purity of the silver electrodes and the combination of electrodes sizes on the yield rate of the produced SNPs. The following main conclusions can be drawn:1. The arc intensity was found to have a significant effect on the SNPs yield rate within the experimental 15 ampere.2. The SNPs production rate increased with the increase in the purity of the silver source material of the electrodes 3. The following conditions have been found to produce highest SNPs rate of 10 g/hr within the experimental limitations of the constructed set up in lab:• 99.9% pure silver electrodes of 1 mm anode and cathode diameters. • 15 ampere and 24 volt submerged arc in de-ionized water passing through 1 mm gap.4. HRTEM images have been used to capture and visualize the different stages of SNPs
References
| [1] | J. Rungby, “The silver nitrate prophylaxis of Crede causes silver deposition in the cornea of experimental animals,” Experimental Eye Research, vol. 42, no. 1, pp. 93–94, 1986. |
| [2] | S. R. Norrby, C. E. Nord, and R. Finch, “Lack of development of new antimicrobial drugs: a potential serious threat to public health,” The Lancet Infectious Diseases, vol. 5, no. 2, pp. 115–119, 2005. |
| [3] | C. Tenover, “The real vancomycin-resistant Staphylococcus aureus has arrived,” Clinical Microbiology Newsletter, vol. 27, no. 5, pp. 35–40, 2005. |
| [4] | N. Ichinose, Y. Ozaki, and S. Kashu, Superfine Particle Technology, Springer, New York, NY, USA, 1992. |
| [5] | Y. Li, P. Leung, L. Yao, Q. W. Song, and E. Newton, “Antimicrobial effect of surgical masks coated with nanoparticles,” Journal of Hospital Infection, vol. 62, no. 1, pp. 58–63, 2006. |
| [6] | See for example: M. G. Bawendi, M. L.Steigerwald and L. E. Brus // Annu. Rev. Phys. Chem. 41 (1990) 477. |
| [7] | T. Sun and K. Seff // Chem. Rev. 94 (1994) 857. |
| [8] | See for example: H. H. Huang, X. P. Ni, G. L.Loy, C. H. Chew, K. L.Tan, F. C. Loh, J. F. Deng and G. Q. Xu // Langmuir 12 (1996) 909. |
| [9] | S.-H. Park, J.-H. Im, J.-W. Im, B.-H. Chun and J.-H. Kim // Microchemical Journal 63 (1999) 71. |
| [10] | See for example: H.-J. Lee, S.-Y. Yeo and S.-H. Jeong // J. Mat. Sci. 38 (2003) 2199. |
| [11] | J.P. Abid, A.W. Wark, P.F. Brevet and H.H. Girault // Chem. Commun. 7 (2002) 792. |
| [12] | G.T. Fei, R. Lu, Z.J. Zhang, G.S. Cheng, L.D. Zhang and P. Cui // Mater. Res. Bull. 32 (1997) 603. |
| [13] | Y.H. Chen and S. Yeh // Colloid. Surf. A 197 (2002) 133. |
| [14] | H. H. Huang, X. P. Ni, G. L. Loy, C. H. Chew, K. L. Tan, F. C. Loh, J. F. Deng and G. Q. Xu // Langmuir 12 (1996) 909. |
| [15] | B. Li, Y. Xie, J. Huang, Y. Liu and Y. Qian // Chem. Mater. 12 (2000), 2614. |
| [16] | Y. Y. Yu, S. S. Chang, C. L. Lee and C. R. C. Wang // J. Phys. Chem. B 101 (1997) 6661. |
| [17] | A. Henglein // J. Phys. Chem. B 104 (2000) 2201. |
| [18] | J.-K. Lung, J.-C. Huang, D.-C. Tien et al., “Preparation of gold nanoparticles by arc discharge in water,” Journal of Alloys and Compounds, vol. 434-435, pp. 655–658, 2007. |
| [19] | C.-H. Lo, T.-T. Tsung, and H.-M. Lin, “Preparation of silver nanofluid by the submerged arc nanoparticle synthesis system (SANSS),” Journal of Alloys and Compounds, vol. 434-435, pp. 659–662, 2007. |
| [20] | Liang-Chia Chen, “Investigation on morphology measurement and evaluation of TiO2nanoparticles synthesized by SANSS,” Journal of Alloys and Compounds, vol. 483, no. 1-2, pp. 366–370, 2009. |
| [21] | Liang-Chia Chen, “Preparation of TiO2 nanoparticles by submerged arc nanoparticle synthesis system,” Journal of Alloys and Compounds, vol. 495, no. 2, pp. 476–480, 2010. |
| [22] | D. C. Tien, C. Y. Liao, Y. C. Chen, et al., “Ionic concentration of sanns colloidal silver and antimicrobial effect on Staphylococcus aureus,” in Proceedings of the International Symposium on Biomedical Engineering (ISOBME '06), Taipei, Taiwan, December 2006. |
| [23] | Q. L. Feng, J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim, and J. O. Kim, “A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus,” Journal of Biomedical Materials Research, vol. 52, no. 4, pp. 662–668, 2000. |
| [24] | T.T. Tsung, H. Chang, L.C. Chen, L.L. Han, C.H. Lo and M.K. Liu // Mater. Tran. The Japan Institute of Metals, 44 (2003), 1138. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML