-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2013; 3(3): 48-51
doi:10.5923/j.nn.20130303.03
New Route for Synthesis Magnetite Nanoparticles from Ferrous Ions and Pistachio Leaf Extract
Nidá M. Salem1, Ahmad L. Ahmad2, Akl M. Awwad2
1Department of Plant Protection, Faculty of Agriculture, Jordan University, Amman, Jordan
2Royal Scientific Society, El Hassan Science City, Amman, Jordan
Correspondence to: Akl M. Awwad, Royal Scientific Society, El Hassan Science City, Amman, Jordan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Magnetite nanoparticles have been successfully synthesized in pistachio leaf extract at ambient temperature. Pistachio leaf extract plays a crucial role in partial oxidation of Fe(II) ions, and determining the morphology and colloidal stability of the resulting magnetite nanoparticles. The synthesized magnetite nanoparticles were characterized by Fourier transforms infrared spectrophotometer (FT-IR), scanning electron microscopy (SEM) and X-ray diffraction (XRD). XRD analysis showed that the synthesized magnetite nanoparticles are highly crystalline and well-monodisperse with 4.8 nm of average diameter. Nanoparticles size was controlled in the range 5-12 nm by the amount of pistachio leaf extract in one-pot reaction at ambient temperature.
Keywords: MagnetiteNanoparticles, Pistachio Leaf Extract, Ferrous Ions
Cite this paper: Nidá M. Salem, Ahmad L. Ahmad, Akl M. Awwad, New Route for Synthesis Magnetite Nanoparticles from Ferrous Ions and Pistachio Leaf Extract, Nanoscience and Nanotechnology, Vol. 3 No. 3, 2013, pp. 48-51. doi: 10.5923/j.nn.20130303.03.
Article Outline
1. Introduction
- Various approaches for synthesis magnetite nanoparticles have been developed such as chemical precipitation method, which involves co-precipitation of ferric (III) and ferrous (II) ions by sodium hydroxide (NaOH) or ammonia solution (NH3.H2O)[1-3], micro-emulsion[4,5], solvothermal[6-8], thermal decomposition[9,10], electrochemical synthesis [11,12], sol-gel method[13], hydrothermal synthesis[14] and ultrasonic technique[15]. These methods have many disadvantages due to the difficulty of scale up of the process, separation and purification of nanoparticles from the oil, surfactant, co-surfactant, and organic solvents. The physical and chemical properties of magnetite nanoparticles are greatly affected by the synthesis route. For this reason, various methods have been reported in the literature for the chemical modification of the magnetite particle surface to obtain the functionalized magnetic nanoparticles. Many studies realized this process by using aminoacids[16], phenol[17], polysulfonic acid[18], 1-hydroxybenzotriazole hydrate and 1-ethyl (dimethyl lamino) propyl carbodiimide[19], 2-pyrrolidone and carboxyl-terminated poly (ethylene glycol)[20], sodium oleate, toluene and ethanol[21], phenyl ether, oleic acid and oleylamine[22], poly (ethylene glycol -700) diacrylate, acrylic acid, Tween-20 and 2-hydroxy-2-methylpropiophenon[23], and polyols[24]Developing facile and green methodsforsynthesizing magnetite nanoparticles are of importance and still a challenge for materials researchers. Over the past decade, there have been increased emphases on the topic of green chemistry. Utilization of non-toxic and eco-fiendally materials are the key issues that merit importance consideration in developing green chemistry. In this research work, we have developed a new and green route for synthesis magnetite nanoparticles from ferrous chloride tetrahydrate and pistachio leaf extract at room temperature.
2. Experimental Section
2.1. Preparation of Pistachio Leaf Extract (PLE)
- Freshly pistachioleaves were collected from pistachio trees planted at the campus of the Royal Scientific Society, Jordan and washed several times with distilled water to remove the dust particles and then sun dried to remove the residual moisture. The pistachioleaves extract used for synthesis magnetite nanoparticles was prepared by placing 1 g of washed dried fine cut leaves in 250 mL glass beaker along with 100 mL of sterile distilled water. The mixture was then boiled for 5 minutes, the colour of the aqueous solution changed from watery to light yellow color. Then the extract was cooled to room temperature and filtered with Whatman No. 1 filter paper before centrifuging at 1000 rpm for 2 minutes to remove the heavy biomaterials. The extract was stored at room temperature in order to be used for further experiments.
2.2. Synthesis of Magnetite Nanoparticles
- 0.52 g of ferrous chloride tetrahydrate (FeCl2.4H2O, E Merck) was dissolved in 100 ml sterile deionized water in a 250 mL glass conical flask and stirred magnetically at room temperature and atmospheric pressure.Afterwards, 50 mL of pistachio leaf extract was added, immediately the yellow color of ferrous chloride tetrahydrate changed to green color, indicating the partially oxidation of ferrous ions to ferric ions, which becomesa mixture of Fe(II) and Fe(III).Within 1 minutes the green mixture under stirring changed to black color, indicating the formation of magnetite nanoparticles, Figure 1. The suspended black particles solution was purified by dispersing in sterile distilled water and centrifugation three times. The magnetite particles are divided into two parts. In the first part, the magnetite nanoparticles are remained in the sterile deionized water without any additives as prepared. The stability of magnetite suspended nanoparticles in sterile deionized water for more than two weeks. In the second part, the magnetite nanoparticles after purification were dried under vacuum. The solid sample was used for further characterization.
2.3. Characterization
- Magnetite (Fe3O4) nanoparticles synthesized by this green method were examined by X-ray diffractometer, (XRD-6000, Shimadzu, Japan) equipped with Cu K α radiation source (λ =0.154056 nm) using Ni as filter at a setting of 30 kV/30mA.All XRD data were collected under the experimental conditions in the angular range 3o≤2θ ≤ 50o.FT-IR spectra of solid powder of pistachio leaf extract and synthesized magnetite nanoparticles were obtained in the range 4000-400 cm-1 with IR-Prestige 21 spectrophotometer (Shimadzu, Japan) using KBr pellet method. Scanning electron microscopy (SEM) images were taken using a field emission scanning electron microscopy (Hitachi S4700, 15 kV).
3. Results and Discussion
3.1. X-ray Diffraction (XRD) and SEM Analysis
- Analysis through X-ray diffraction was carried out to confirm the crystalline nature of the magnetite nanoparticles. Fig. 2 shows the X-ray diffraction (XRD) patterns of the magnetite nanoparticles were synthesized by pistachio leaf extract. The nanoparticles are well-crystalline and the position and relative intensity of the diffraction peaks match well withthe standardXRD data for bulk magnetite (JCPDS file No. 19-0629). The 2θ peaks at 30.2o, 35.52o, 43.54o, 54.21o, 56.85o and 62.84o are attributed to the crystal planes of magnetite at 220, 311, 400, 422, 511 and 440, respectively. The X-ray diffraction results clearly show that the magnetite nanoparticles formed by our green method using pistachio leaf extract are crystalline in nature. The peak broading of XRD patterns indicates the significantly small size of the resulting crystallites. The average crystallite sizes of the synthesized magnetite nanoparticles by our simple green method calculated by Debye-Scherer equation[25]:D = Kλ / β cos θ Where D = the crystallite size of AgNPs particlesλ = the wavelength of x-ray source (0.1541 nm) used in XRD β = the full width at half maximum of the diffraction peak. K = the Scherrer constant with value from 0.9 to 1. θ = the Bragg angle. The value 4.8 nmobtained from XRD data are very close to the value 6.2 nm ± 1.4 nm of the mean particle sizes determined by SEM image, Figure 3
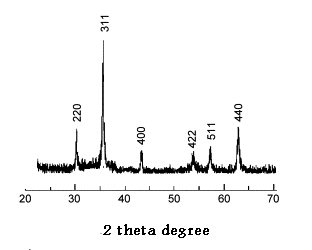 | Figure 2. XRD pattern of the synthesized magnetite nanoparticles |
 | Figure 3. SEM image of synthesized magnetite nanoparticles |
3.2. Surface Charge and Surface Coating
- The FT-IR spectra of pistachio leaf extract is given in Fig. 4A.A broad and strongband 3387 cm-1 is due to the N-H stretching, bending vibration of amine group NH2 and O-H the overlapping of the stretching vibration of attributed for water and pistachio leaf extract molecules. The peaks at 2912 cm-1 and 2843 cm-1 could be assigned to the stretching vibrations of –CH3 and CH2 functional groups. The strong band at 1612 cm-1isidentified as the amide I and amide II, which arise due to C=O and NH stretching vibrations in the amide linkage of the protein.The peaks at 1384 cm-1 and 12123 cm-1 can be assigned to the C-O group of polyols. The peaks at around 1072 cm-1 corresponds to C-N stretching vibrations of aliphatic amines. FT-IR spectra of synthesized magnetite nanoparticles were carried out to identify the possible biomolecules responsible for oxidation of Fe (II) to Fe(III), capping and stabilization of nanoparticles. Fig. 4B shows the peaks associated with magnetite nanoparticles. The peaks 3417 cm-1 and1612 cm-1have been assigned toO-H stretching,N-H stretching and bending vibration of amine NH2 group in pistachio leaf extract and the overlap of the stretching vibration of O-H.The peak at 1338 cm-1 is attributed to the asymmetric and symmetric stretching vibration of COO-. The presence of magnetite nanoparticles can be seen by two absorption bands at around 571 cm-1 and 432 cm-1 which, corresponding to the Fe-O stretching band of bulk magnetite (Fe3O4). These results revealed that the COO- groups were bonded on the magnetite particle surface. Overall the observation confirms the presence of protein in pistachio leaf extract, which acts as oxidizing as wel as capping agent and stabilizer for magnetite nanoparticles.
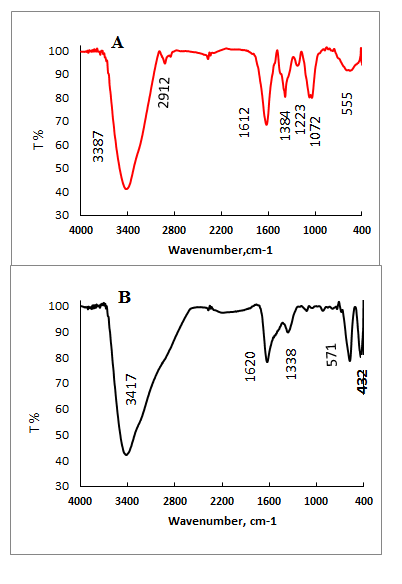 | Figure 4. FT-IR spectra of pistachio leaf extract (A) and magnetite nanoparticles (B) |
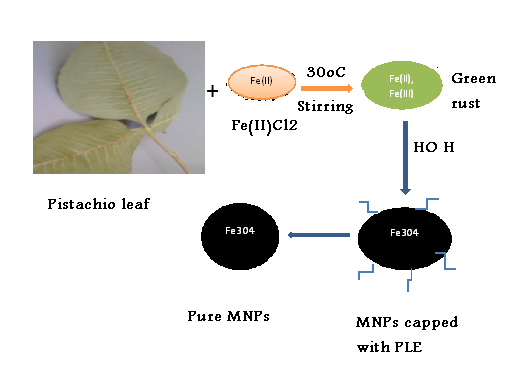 | Scheme 1. The proposed formation mechanism of magnetite nanoparticles |
4. Conclusions
- A new and green route have been developedfor synthesis magnetite nanoparticles in one-pot reaction process at ambient temperature. Our synthesis approach employed an environmental friendly biomaterial, pistachio leaf extract, as an alternative to organic solvents and surfactants. Pistachio leaf extract acts as an oxidizing agent for ferrous ions to ferric ions, dispersing and stabilizing agent, which prevents the agglomeration of magnetite nanoparticles (Fe3O4) formed during synthesis. XRD and SEM data showed that the size and shape of the Fe3O4 nanoparticles could be controlled by the amount of pistachio leaf extract and the concentration of Fe(II). The method in the present study offers several important advantageous features. First, the synthesis method is economical and environmentally friendly, because it involves inexpensive and non-toxic materials. Second, size-controlled Fe3O4 nanoparticles are produced easily by different amounts of pistachio leaf extract.
ACKNOWLEDGMENTS
- The authors would like to thank Abdul HameedShoman Fund for Support of Scientific Research, Jordan for financial support of this work. We are also thankful to Royal Scientific Society and Jordan University, Jordanfor providing all facilities to carry out this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML