-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2013; 3(3): 35-39
doi:10.5923/j.nn.20130303.01
A Novel Approach for Synthesis Magnetite Nanoparticles at Ambient Temperature
1Department od Plant Protection, Faculty of Agriculture, Jordan University, Amman, Jordan
2Department of Knowledge, Royal Scientific Society, El Hassan Science City, Amman, Jordan
Correspondence to: Akl M. Awwad, Department of Knowledge, Royal Scientific Society, El Hassan Science City, Amman, Jordan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A novel and facile approach for synthesis magnetite (Fe3O4) nanoparticles from ferric chloride (FeCl3) and ferrous chloride tetrahydrate (FeCl2.4H20) in the presence of pistachio leaf extract at ambient temperature and atmospheric pressure. The effect of pistachio leaf extract amount in the reaction mechanism and particle size was studied. The synthesized magnetite nanoparticles were characterized by Fourier transforms infrared spectrophotometer (FT-IR), scanning electron microscopy (SEM) and X-ray diffraction (XRD). XRD analysis showed that the synthesized magnetite nanoparticles are highly crystalline and well-monodisperse with 8 nm of average diameter. The synthesized magnetite nanoparticles can be easily dispersed in aqueous media due to coated by a layer of pistachio leaf extract in situ. This approach provides a facile route to prepare magnetite nanoparticles.
Keywords: Magnetite Nanoparticles, Pistachio Leaf Extract, One-Pot Reaction
Cite this paper: Nidá M. Salem, Akl M. Awwad, A Novel Approach for Synthesis Magnetite Nanoparticles at Ambient Temperature, Nanoscience and Nanotechnology, Vol. 3 No. 3, 2013, pp. 35-39. doi: 10.5923/j.nn.20130303.01.
Article Outline
1. Introduction
- Magnetite (Fe3O4) nanoparticles have received much interest due to its magnetic, catalytic, magnetic recording, drug delivery, magnetic resonance imaging, cancer diagnosis and treatment, bimolecular separation, etc. Various methods for synthesis magnetite nanoparticles have been developed such as micro-emulsion method[1,2], thermal decomposition of organic iron precursors in organic solvents[3-5], co-precipitation process[6,7], sol-gel method [8], solvothermal method[9,11], hydrothermal synthesis [12,13], electrochemical synthesis[14],ultrasonic chemical co-precipitation[15,16]. These methods have many disadvantages due to the difficulty of scale up of the process, separation and purification of nanoparticles from the oil, surfactant, co-surfactant, organic solvents[17-25]. We have attempted to synthesis magnetite nanoparticles by co-precipitation of Fe3+ and Fe2+ ions without using toxic organic solvents and surfactants. In this one-pot synthesis process, magnetite nanoparticles were synthesized by co-precipitation of iron oxides in presence of pistachio leaf extract at ambient temperature and atmospheric pressure. Thereby the approach is economic, green, facile and easily to scale up.
2. Experimental Section
2.1. Materials
- Ferric chloride (FeCl3, Aldrich 98%), ferrous chloride tetra hydrate (FeCl2.4H2O, Aldrich 99%), pistachio leaf extract and sodium hydroxide (NaOH, Aldrich) were used for synthesis of magnetite nanoparticles without further purification as well as deionized sterile water with pH 7.0 and conductivity 1 µS/cm.
2.2. Preparation of Pistachio Leaf Extract
 | Figure 1. A photograph shows the pistachio leaves and their extract |
2.3. Synthesis of Magnetite Nanoparticles
- 100 ml of aqueous mixture containing 0.01 M ferrous chloride tetra hydrate and 0.02 M of anhydrous ferric chloride (1/2 molar ratio) was placed in a 250 mL glass beaker and stirred magnetically at room temperature and atmospheric pressure. Afterwards, 10 mL of an alkaline solution of Pistachio leaf extract (pH = 8.0) was added, the yellowish brown color of iron chlorides changed immediately to black color, indicating the formation of magnetite nanoparticles. The suspended black particles solution was purified by dispersing in sterile distilled water and centrifugation three times. The magnetite particles are divided into two parts. In the first part, the magnetite nanoparticles are remained in the sterile distilled water without any additives as prepared. The stability of magnetite suspended nanoparticles in sterile distilled water for more than four weeks. In the second part, the magnetite nanoparticles after purification were dried under vacuum. The solid sample was used for further characterization.
2.4. Characterization
- Magnetite (Fe3O4) nanoparticles synthesized by this green method were examined by X-ray diffractometer, XRD-6000 (Shimadzu, Japan) equipped with Cu K α radiation source using Ni as filter at a setting of 30 kV/30mA. All XRD data were collected under the experimental conditions in the angular range 3o ≤ 2θ ≤ 50o. FT-IR spectra of Pistachio leaf extract and magnetite nanoparticles were obtained in the range 4000-400 cm-1 with IR-Prestige 21 spectrophotometer (Shimadzu, Japan) using KBr pellet method. Scanning electron microscopy (SEM) analysis of synthesized magnetite nanoparticles was done by S-4500 SEM machine (Hitachi, Japan).
3. Results and Discussion
3.1. X-ray diffraction (XRD) studies
- Analysis through X-ray diffraction was carried out to confirm the crystalline nature of the magnetite nanoparticles. The XRD pattern showed numbers of Braggs reflections that may be indexed on the basis of the face cantered cubic structure of magnetite. A comparison of our XRD spectrum with the standard XRD data for bulk magnetite (JCPDS file No. 19-0629) confirmed that the magnetite particles formed in our experiments were in the form of nanocrystals, as evidenced by the peaks at 2θ values of 18.55o, 30.81o, 36.48o, 43.52o, 54.59o, 56.83o and 62.34o corresponding to (111), (220), (311), (222), (400), (422) and (511) Bragg's reflections, respectively, which may be indexed based on the face centered cubic (fcc) structures of magnetite. The X-ray diffraction results clearly show that the magnetite nanoparticles formed by our co-precipitation method in presence of Pistachio leaf extract are crystalline in nature. The unassigned peaks at 2θ = 31.88o, 37.44o and 46.18o denoted by (*) in Fig. 2 are thought to be related to crystalline and amorphous organic phases, It was found that the average size from XRD data and using Debye-Scherer equation was approximately 8 nm. The presence of structural peaks in XRD patterns and average crystalline size around 8 nm clearly illustrates that magnetite particles synthesized by our green method were nanocrystalline in nature. The average particle size of magnetite nanoparticles synthesized by the present green method can be calculated using Debye-Scherrer equation[26].D = K λ/β cos θWhereD is the mean diameter of nanoparticles, β is the full width at half-maximum value of XRD diffraction lines, λ is the wavelength of X-ray radiation source 0.15405 nm, Θ is the half diffraction angle –Bragg angle and K is the Scherrer constant with value from 0.9 to 1. The presence of structural peaks in XRD patterns and average crystalline size calculated 8 nm clearly illustrates the magnetite nanoparticles synthesized by our green approach were nanocrystalline in nature.
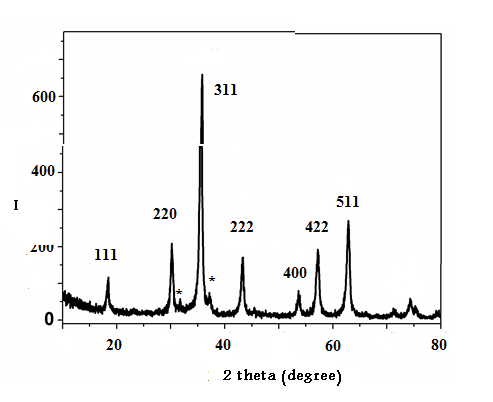 | Figure 2. XRD pattern of the synthesized magnetite nanoparticles |
3.2. Surface Charge and Surface coating
- The FT-IR spectra of Pistachio leaf extract is given in Fig. 3. A broad band between 3417 cm-1 is due to the N-H stretching and bending vibration of amine group NH2 and O-H the overlapping of the stretching vibration of attributed for water and Pistachio leaf extract molecules. The peak at 2920 cm-1 could be assigned to the stretching vibrations of –CH3 and CH2 functional groups. The strong band at 1635 cm-1 and the peak at 1438 cm-1 are identified as the amide I and amide II, which arise due to C=O and NH stretching vibrations in the amide linkage of the protein. The peaks at 1311 cm-1 and 1203 cm-1 can be assigned to the C-O group of polyols. The peak at 1041 cm-1 corresponds to C-N stretching vibrations of aliphatic amines.
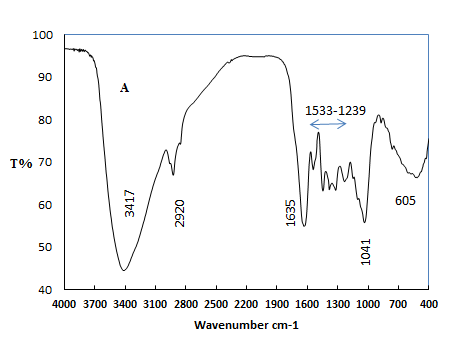 | Figure 3. FT-IR spectra of pistachio leaf extract |
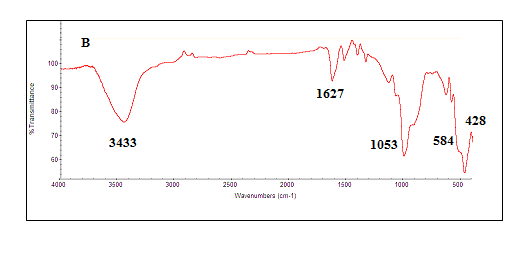 | Figure 4. FT-IR spectra of synthesized magnetite nanoparticles |
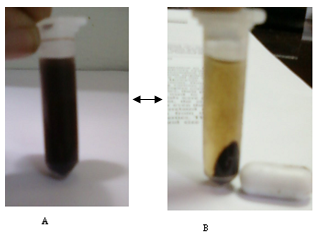 | Figure 5. Photographs of the separation A to B and dispersion B to A of the pistachio magnetite nanoparticles: (A) without external magnetic field and (B) with external magnetic field |
3.3. SEM Analysis of Magnetite Nanoparticles
- The morphology of pistachio leaf extract stabilized magnetite nanoparticles (Fe3O4) have been investigated by scanning electron microscope. Fig. 6 shows the scanning electron microscope (SEM) images of MNPs prepared by this green method in presence of Pistachio leaf extract. It can be clearly seen from SEM images that MNPs are nearly spherical with an average diameter ranges from 5-18 nm. The size and shape of the synthesized magnetite nanoparticles could be controlled by the amount of Pistachio leaf extract and the concentration of Fe3+/Fe2+. The crystalline and organic phases of pistachio leaf extract are shown in The SEM image with MNPS denoted by red circles, which acts as stabilizing agent for magnetite nanoparticles and preventing agglomeration of particles. This research work is the first to report on using Pistachio leaf extract to synthesis monodisperse magnetite nanoparticles via co-precipitation method in the presence of Pistachio leaf extract, as illustrated in scheme 1. By changing the amount of pistachio leaf extract, the magnetite nanoparticles sizes could be adjusted from 5 ± 1.2 to 18 ± 2.2 nm.
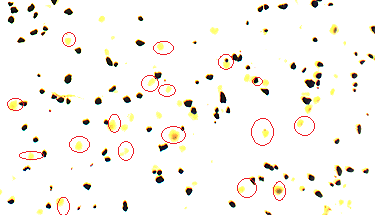 | Figure 6. SEM images of synthesized magnetite nanoparticles |
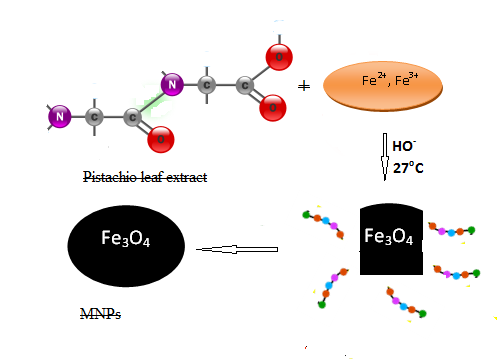 | Scheme 1. Synthesis route of magnetite nanoparticles |
4. Conclusions
- A novel and simple approach for synthesis magnetite nanoparticles in one-pot reaction process at ambient temperature and atmospheric pressure. Our synthesis approach employed an environmental friendly solvent, pistachio leaf extract, as an alternative to organic solvents and surfactants. Pistachio leaf extract acts both as dispersing and stabilizing agent, which prevents the agglomeration of magnetite nanoparticles (Fe3O4) formed during synthesis. XRD and SEM data showed that the size and shape of the Fe3O4 nanoparticles could be controlled by the amount of Pistachio leaf extract and the concentration of Fe3+ and Fe2+. The method in the present study offers several important advantageous features. First, the synthesis method is economical and environmentally friendly, because it involves inexpensive and non-toxic materials. Second, size-controlled Fe3O4 nanoparticles are produced easily by different amounts of Pistachio leaf extract.
ACKNOWLEDGMENTS
- The authors would like to thank Abdul Hameed Shoman Fund for Support of Scientific Research (AHSFSSR), Jordan for financial support. We are also thankful to Royal Scientific Society and Jordan University, Jordan for providing all facilities to carry out this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML