| [1] | (a) A. P. Alivisatos, Science, 1996, 271, 933; (b) H. Weller, Angew.Chem., Int. Ed. Engl., 1993, 32, 41; (c) Clusters and Colloids, ed. G. Schmid, VCH Press, New York, 1994; (d) Nanoscale Materials in Chemistry, ed. K. J. Klabunde, Wiley-Interscience, New York, 2001; (e) Nanoparticles and Nanostructured Films, ed. J. H. Fendler, Wiley- VCH, Weinheim, 1998). |
| [2] | Morteza Mahmoudi, Shilpa Sant, Ben Wang, Sophie Laurent, Tapas Sen. Advanced Drug Delivery Reviews (6)3 (2011) 24–46. |
| [3] | A. Tari, R.W. Chantrell, S.W. Charles, J. Popplewell. Physica B & C 97 (1) (1979) 57–64. |
| [4] | P. Poizot, S. Laruelle, S. Grugeon, L. Dupont, J.M. Tarascon. Nature 407 (6803) (2000) 496–499. |
| [5] | M. Mahmoudi, A. Simchi, M. Imani, P. Stroeve, A. Sohrabi.Thin Solid Films 518 (15) (2010) 4281–4289. |
| [6] | (a) D. D. Awschalom and D. P. DiVicenzo, Phys. Today, 1995, 4, 43; (b) K. Raj and R. Moskowitz, J. Magn. Magn. Mater. 1990, 85, 233; (c) I.M. L. Billas, A. Chatelain and W. A. de Heer, Science, 1994, 265, 1682; (d) R. C. O’Handley, Modern Magnetic Materials, Wiley, New York,1999). |
| [7] | Rudolf Hergt, Silvio Dutz, Robert M¨uller and Matthias Zeisberge. Chemcomm.rsc.org December 2002. |
| [8] | Batlle X and Labarta A 2002 J. Phys. D: Apply. Phys. 35 R15. |
| [9] | Frenkel J and Dorfman J 1930 Nature 126- 274. |
| [10] | The Physics and Chemistry of Nanosolids Wiley. FrankJ.Owens, Charles P.PooleJr.2009. |
| [11] | Bean C P and Livingston J D 1959 J. Appl. Phys. 30 120. |
| [12] | Babes L, Denizot B, Tanguy G, Le Jeune JJ, Jallet P.a parametricstudy. J Coll Int Sci 1999)(212) 474–82. |
| [13] | Goya GF, Berquo TS, Fonseca FC. J ApplPhys 2003;94(5):3520–8. |
| [14] | Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, Frank JA. Radiology 2003;229 (3):838–46. |
| [15] | Reimer P, Weissleder R. Radiology 1996;36:153–63. |
| [16] | Pankhurst QA, Connolly J, Jones SK, Dobson J. J Phys D: Appl Phys 2003;36:R167–81. |
| [17] | Ha¨ feli U, Schu¨ tt W, Teller J, Zborowski M. New York:Plenum Press; 1997. |
| [18] | Dong-Hwang Chen∗, Shih-Hung Huang. Process Biochemistry 39 (2004) 2207–2211. |
| [19] | Suber L, Foglia S, Ingo GM, Boukos N. Organomet Chem 2001;15:414–20. |
| [20] | Šafaˇr´ık I. Water Res 1995;29:101–5. |
| [21] | Šafaˇr´ık I, Šafa´r´ıková M. Water Res 2002;36:196–200. |
| [22] | Denizli A, Say R. J Biomater Sci Polym Edn 2001;12:1059–73. |
| [23] | Schütt W, Grüttner C, Häfeli U, Zborowski M, Teller J, Putzar H, et al.a mini-review. Hybridoma 1997;16:109–17. |
| [24] | Häfeli U, Schütt W, Teller J, Zborowski M. New York: Plenum Press, 1997. |
| [25] | Xu Z, Liu Q, Finch JA. New York: Marcel Dekker, 1999. p. 31–50. |
| [26] | Ying-Sing Li a, Jeffrey S. Church, Andrea L. Woodhead , Filsun Moussa . Spectrochimica Acta Part A 76 (2010) 484–489. |
| [27] | P. Majewski, B. Thierry, Critical Reviews in Solid State and Material Sciences 32 (3-4) (2007) 203-215. |
| [28] | Sugimoto T 2000 Fine Particles: Synthesis, Characterisation and Mechanism of Growth (New York: Marcel Dekker) |
| [29] | Bing Tanga, Liangjun Yuana, Taihong Shib, Linfeng Yua, Youchun Zhua. Journal of Hazardous Materials 163 (2009) 1173–1178. |
| [30] | LaMer V K and Dinegar R H 1950 J. Am. Chem. Soc. 72 4847. |
| [31] | T. Sugimoto, E. Matijevic, Journal of Colloid and Interface Science 74 (1) (1980) 227-243. |
| [32] | A. Chastellain, A. Petri, H. Hofmann, Journal of Colloid and Interface Science 278 (2) (2004) 353-360. |
| [33] | Jolivet J P 2000 Metal Oxide Chemistry and Synthesis: From Solutions to Solid State (New York: Wiley) |
| [34] | P. Tartaj, M.D. Morales, S. Veintemillas-Verdaguer, T. Gonzalez-Carreno, C.J. Serna, Journal of Physics D:Applied Physics 36 (13) (2003) R182-R197. |
| [35] | P. Tartaj, M.P. Morales, T. Gonzalez-Carreno, S. Veintemillas-Verdaguer, C.J. Serna, Journal of Magnetism and Magnetic Materials 290 (2005) 28-34. |
| [36] | D.K. Kim, Y. Zhang, W. Voit, K.V. Rao, M. Muhammed, Journal of Magnetism and Magnetic Materials 225 (1-2) (2001) 30e36. |
| [37] | D.K. Kim, M. Mikhaylova, Y. Zhang, M. Muhammed, Chemistry of Materials 15 (8) (2003) 1617-1627. |
| [38] | C.L. Lin, C.F. Lee, W.Y. Chiu, Journal of Colloid and Interface Science 291 (2) (2005) 411-420. |
| [39] | Z.P. Xu, Q.H. Zeng, G.Q. Lu, A.B. Yu, Chemical Engineering Science 61 (3) (2006) 1027-1040. |
| [40] | H. Lee, E. Lee, D.K. Kim, N.K. Jang, Y.Y. Jeong, S. Jon, Journal of the American Chemical Society 128 (22) (2006) 7383-7389. |
| [41] | S. Mohapatra, N. Pramanik, S. Mukherjee, S.K. Ghosh, P. Pramanik, Journal of Materials Science 42 (17) (2007) 7566-7574. |
| [42] | D.K. Kim, M. Mikhaylova, F.H. Wang, J. Kehr, B. Bjelke, Y. Zhang, T. Tsakalakos, M. Muhammed, Chemistry of Materials 15 (23) (2003) 4343-4351. |
| [43] | Bergemann C, M¨uller-Schulte D, Oster J, Brassard L and L¨ubbe A S 1999 J. Magn. Magn. Mater. (194)(45). |
| [44] | R.M. Cornell, U. Schwertmann, The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, second ed. Wiley-VCH, Weinheim, 2003. |
| [45] | Sophie Laurent,Delphine Forge, Marc Port,Alain Roch, Caroline Robic, Luce Vander Elst,†and Robert N. Muller. Chem. Rev. 2008, 108, 2064–2110. |
| [46] | Massart, R. IEEE Trans. Magn. 1981, 17, 1247. |
| [47] | Massart, R.; Cabuil, V. J. Chim. Phys. 1987, 84, 7. |
| [48] | Gribanow, N. M.; Bibik, E. E.; Buzunov, O. V.; Naumov, V. N. J. Magn. Magn. Mater 1990, 85, 7. |
| [49] | A. Tavakoli, M. Sohrabi, A. Kargari, Chemical Papers (2007) 61 (3) 151-170. |
| [50] | Y.X. Pang, X.J. Bao, Journal of Materials Chemistry 12 (12) (2002) 3699-3704. |
| [51] | V. Pillai, P. Kumar, M.J. Hou, P. Ayyub, D.O. Shah, Advances in Colloid and Interface Science 55 (1995)241-269. |
| [52] | P. Tartaj, L.C. De Jonghe, Journal of Materials Chemistry 10 (12) (2000) 2786-2790. |
| [53] | I. Capek, Advances in Colloid and Interface Science 110 (1-2) (2004) 49-74. |
| [54] | Pileni M P 1993 J. Phys. Chem. (97) (6961). |
| [55] | Salazar-Alvarez, G. Doctoral Thesis, Stockholm, Sweden, 2004. |
| [56] | Pedro Tartaj1, Mar´ıa del Puerto Morales1,Sabino Veintemillas-Verdaguer, Teresita Gonz´alez-Carre ˜no and Carlos J Serna (2003). J. Phys. D: Appl. Phys. 36 R182–R197 |
| [57] | Dai, Z.; Meiser, F.; Mo¨hwald, H. J. Colloid Interface Sci. 2005, 28 (1), 298. |
| [58] | Dura˜es, L.; Costa, B. F. O.; Vasques, J.; Campos, J.; Portugal, A. Mater. Lett. 2005, 59 (7) 859. |
| [59] | Ismail, A. A. Appl. Catal., B 2005, 58, 115. |
| [60] | U.T. Lam, R. Mammucari, K. Suzuki, N.R. Foster, Industrial & Engineering Chemistry Research 47 (3) (2008) 599-614. |
| [61] | Liu, X. Q.; Tao, S. W.; Shen, Y. S. Sens. Actuators, A 1997. 40, 161. |
| [62] | Kojima, K.; Miyazaki, M.; Mizukami, F.; Maeda, K. J. Sol-Gel Sci. Technol. 1997, 8, 77. |
| [63] | Cannas, C.; Gatteschi, D.; Musinu, A.; Piccaluga, G.; Sangregorio, C. J. Phys. Chem. 1998, 102, 7721. |
| [64] | Ennas, G.; Musinu, A.; Piccaluga, G.; Zedda, D.; Gatteschi, D.; Sangregorio, C.; Stanger, J. L.; Concas, G.; Spano, G. Chem. Mater. 1998, 10, 495. |
| [65] | Brinker, C. J.; Sherrer, G. W. Sol-Gel Science; Academic Press: New York, 1990. |
| [66] | da Costa, G. M.; De Grave, E.; de Bakker, P. M. A.; Vandeberghe, R. E. J. Solid State Chem. 1994, 113, 405. |
| [67] | A. Tavakoli, M. Sohrabi, A. Kargari, Chemical Papers 61 (3) (2007) 151-170. |
| [68] | Raileanu, M.; Crisan, M.; Petrache, C.; Crisan, D.; Jitianu, A.; Zaharescu, M.; Predoi, D.; Kuncser, V.; Filoti, G. Rom. J. Phys. 2005, 50 (5-6), 595. |
| [69] | M. Tadic, D. Markovic, V. Spasojevic, V. Kusigerski, M. Remskar, J. Pirnat, Z. Jaglicic, Journal of Alloys and Compounds 441 (1-2) (2007) 291-296. |
| [70] | Z.Z. Xu, C.C. Wang, W.L. Yang, S.K. Fu, Journal of Materials Science 40 (17) (2005) 4667- 4669. |
| [71] | Y.H. Deng, C.C. Wang, J.H. Hu, W.L. Yang, S.K. Fu, Colloids and Surfaces A: Physicochemical and Engineering Aspects 262 (1-3) (2005) 87-93. |
| [72] | C.T. Wang, R.J. Willey, Journal of Non-Crystalline Solids 225 (1) (1998) 173-177. |
| [73] | C.-T. Wang, S.-H. Ro, Applied Catalysis A: General 285 (1e2) (2005) 196-204. |
| [74] | A. Tavakoli, M. Sohrabi, A. Kargari, Chemical Papers 61 (3) (2007) 151-170. |
| [75] | G. Ennas, A. Musinu, G. Piccaluga, D. Zedda, D. Gatteschi, C. Sangregorio, J.L. Stanger, G. Concas, G. Spano,Chemistry of Materials 10 (2) (1998) 495-502. |
| [76] | S. Bruni, F. Cariati, M. Casu, A. Lai, A. Musinu, G. Piccaluga, S. Solinas, Nanostructured Materials 11 (5) (1999) 573-586. |
| [77] | Erin Camponeschi, Jeremy Walker, Hamid Garmestani. ACS Symposium Series; American Chemical Society: Washington, DC, 2008. |
| [78] | Pecharroman, C.; Gonzalez-Carreno, T.; Iglesias, J. E. Phys. Chem.Miner. 1995, 22, 21. |
| [79] | Gonzalez-Carreno, T.; Morales, M. P.; Gracia, M.; Serna, C. J. Mater. Lett. 1993, 18, 151. |
| [80] | A. Tavakoli, M. Sohrabi, A. Kargari, Chemical Papers 61 (3) (2007) 151-170. |
| [81] | S. Martelli, A. Mancini, R. Giorgi, R. Alexandrescu, S. Cojocaru, A. Crunteanu, I. Voicu, M. Balu, I. Morjan, Applied Surface Science 154-155 (2000) 353-359. |
| [82] | R. Alexandrescu, I. Morjann, A. Crunteanu, S. Cojocaru, S. Petcu, V. Teodorescu, F. Huisken, B. Koh, M. Ehbrecht, Materials Chemistry and Physics 55 (2) (1998) 115-121. |
| [83] | I. Morjan, R. Alexandrescu, I. Soare, F. Dumitrache, I. Sandu, I. Voicu, A. Crunteanu, E. Vasile, V. Ciupina, S. Martelli, Materials Science and Engineering: C 23 (1e2) (2003) 211-216. |
| [84] | F. Dumitrache, I. Morjan, R. Alexandrescu, V. Ciupina, G. Prodan, I. Voicu, C. Fleaca, L. Albu, M. Savoiu, I. Sandu, E. Popovici, I. Soare, Applied Surface Science 247 (1-4) (2005) 25-31. |
| [85] | P. Tartaj, M.P. Morales, T. Gonzalez-Carreno, S. Veintemillas-Verdaguer, C.J. Serna, Journal of Magnetism and Magnetic Materials 290 (2005) 28-34. |
| [86] | P. Tartaj, M.D. Morales, S. Veintemillas-Verdaguer, T. Gonzalez-Carreno, C.J. Serna, Journal of Physics D: Applied Physics 36 (13) (2003) R182-R197. |
| [87] | Benjamin M.Kumfer, KozoShinoda , BalachandranJeyadevan , IanM.Kennedy, Journal of Aerosol Science 41(2010)257–265 |
| [88] | Christoph Schweiger, ClemensPietzonka, JohannesHeverhagen, ThomasKissel . International Journal of Pharmaceutics (2011) (Article in press). |
| [89] | Fievet F, Lagier J P, Blin B, Beaudoin B and Figlarz M 1989.Solid State Ion. 32/33 198. |
| [90] | Je´ze´quel, D.; Guenot, J.; Jouini, N.; Fie´vet, F. J. Mater. Res. 1995,10, 77. |
| [91] | Joseyphus, R. J.; Kodama, D.; Matsumoto, T.; Sato, Y.; Jeyadevan, B.; Tohji, K. J. Magn. Magn. Mater. 2007, 310 (2), 2393. |
| [92] | Hong-Ling Liua, Seung Pil Kob, Jun-Hua Wuc,, Myung-Hwa Jungd, Ji Hyun Minb, Ju Hun Leeb, Boo Hyun Anb, Young Keun Kimb, Journal of Magnetism and Magnetic Materials 310 (2007) 815–817 |
| [93] | B.D. Cullity, S.R. Stock, in: Elements of X-ray Diffraction, Prentice Hall, Englewood Cliffs, New Jersey, 2001. |
| [94] | S. Sun, H. Zeng, J. Am. Chem. Soc. 124 (2002) 8204. |
| [95] | S. Si, A. Kotal, T.K. Mandal, S. Giri, H. Nakamura, T. Kohara,Chem. Mater. 16 (2004) 3489. |
| [96] | A. Tavakoli, M. Sohrabi, A. Kargari, Chemical Papers 61 (3) (2007) 151-170. |
| [97] | Y.L. Hao, A.S. Teja, Journal of Materials Research 18 (2) (2003) 415-422. |
| [98] | C. Xu, A.S. Teja, Journal of Supercritical Fluids 44 (1) (2008) 85-91. |
| [99] | C. Xu, J. Lee, A.S. Teja, Journal of Supercritical Fluids 44 (1) (2008) 92-97. |
| [100] | Osuna, J.; de Caro, D.; Amiens, C.; Chaudret, B.; Snoeck, E.; Respaud, M.; Broto, J. M.; Fert, A. J. Phys. Chem. 1996, 10. |
| [101] | Park, S. J.; Kim, S.; Lee, S.; Khim, Z. G.; Char, K.; Hyeon, T. J. Am. Chem. Soc. 2000, 122, 8581. |
| [102] | Amir Hassanjani-Roshan, Mohammad Reza Vaezi, Ali Shokuhfarc, Zohreh Rajabali. Particuology 9 (2011) 95–99. |
| [103] | Ashok kumar, M., Lee, J., Kentish, S., & Grieser, F. (2007). Bubbles in an acoustic field: An overview. Ultrasonics Sonochemistry, 14, 470–475. |
| [104] | Suslick, K. S. (1998). Sonochemistry, in Kirk–Othmer encyclopedia of chemical technology (4th ed.) John Wiley & Sons: New York. , pp. 517–541. |
| [105] | Wang, X. K., Chen, G. H., & Guo, W. L. (2003). Sonochemical degradation of kinetics of methyl violet in aqueous solutions. Molecules, 8, 40–44. |
| [106] | Mason, T. J., & Lorimer, J. P. (2002). Applied sonochemistry: Uses of power ultrasound in chemistry and processing.Weinheim:Wiley-VCH. |
| [107] | Suslick, K. S., Didenko, Y., Fang, M. M., Hyeon, T., Kolbeck, K. J., McNamara, W. B., III, et al. (1999).Acoustic cavitation and its chemical consequences. Philosophical Transactions of the Royal Society A, 357, 335–353. |
| [108] | Jiri Pinkas , Vendula Reichlova , Radek Zboril , Zdenek Moravec ,Petr Bezdicka c, Jirina Matejkova .Ultrasonics Sonochemistry 15 (2008) 257–264. |
| [109] | R. Gedye, F. Smith, K. Westaway, H. Ali, L. Baldisera, L. Laberge, J. Rousell, Tetrahedron Lett. 27 (1986) 279. |
| [110] | R.J. Giguere, T.L. Bray, S.M. Duncan, G. Majetich, Tetrahedron Lett. 27 (1986) 4945. |
| [111] | Norihito Kijima, Masashi Yoshinaga, Junji Awaka, Junji Akimoto. Solid State Ionics (2011) (Article in press). |
| [112] | J.G. Parsons, C. Luna, C.E. Botez, J. Elizalde, J.L. Gardea-Torresdey. Journal of Physics and Chemistry of Solids 70 (2009) 555–560. |

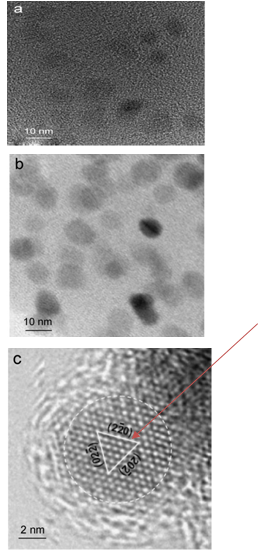
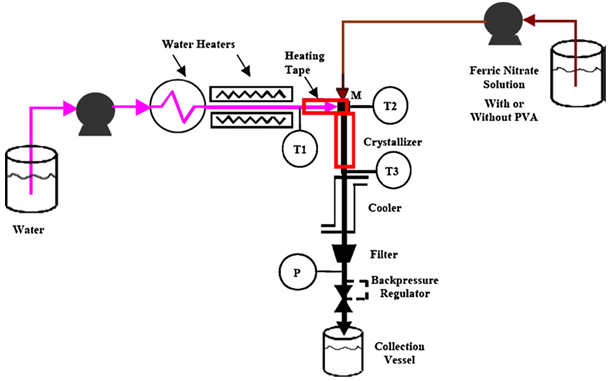
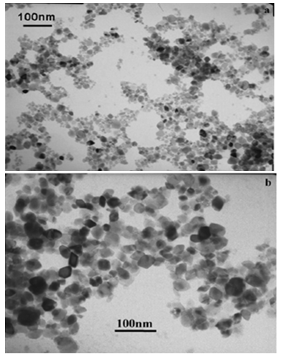
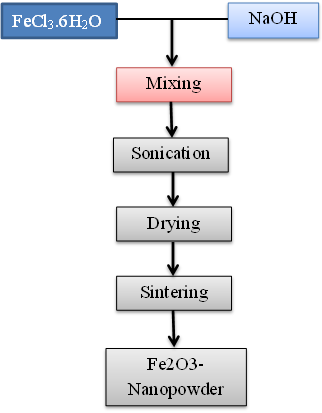
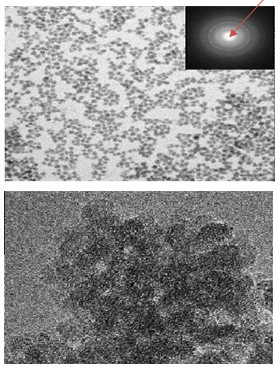
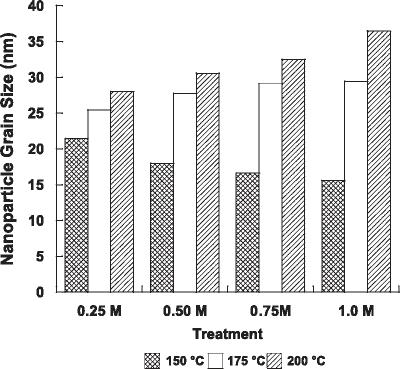
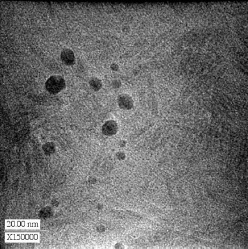
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML