-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 22163-257X e-ISSN: 2163-2588
2012; 2(4): 129-133
doi: 10.5923/j.nn.20120204.07
Experimental Analysis of the Influence of Inert Nano-additives upon Combustion of Diesel Sprays
Giulio Solero
Politecnico di Milano, Department of Energy , via Lambruschini, 4 – Milano
Correspondence to: Giulio Solero , Politecnico di Milano, Department of Energy , via Lambruschini, 4 – Milano.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Recent experimental studies report that addition of nanoparticles (tipically metal oxides nanopowders, such as Al2O3) can somehow modify the ignition mechanism of liquid fuels, probably influencing and accelerating the thermal exchange process between the fuel droplets and the surrounding air.In this paper it has been experimentally analised a stationary Diesel spray, produced by a gas assisted atomizer, preliminarily comparing the behaviour of the flame generated using the unseeded fuel with that one of the same fuel additivated with Al2O3 nanoparticles. Results obtained through light emission analysis are encouraging, in the sense that the presence of a small nano-additive quantity (0.1% in volume) seems to produce effects similar to a shift towards higher flame ventilation, with a possible positive consequence upon combustion stability and efficiency.
Keywords: Nano-Additives, Diesel Spray, Combustion
Article Outline
1. Introduction
- Nanostructured materials represent nowadays a wide and probably still largely unexplored field of potential applications[1]. In fact, this is a research topic in high and rapid development, both at a basic level and under the point of view of possible practical applications, leaving large space for a thorough scientific analysis, which requires with no doubt long time for ultimate conclusions.The potential application fields of nanoparticles are very wide and, probably, not yet fully understood: from energetics (sensors, propulsion, reduction of environmental impact) to chemistry (catalysis, additives) to medicine (diagnostics) and of course to material science. Also the problem of nanoparticles impact on human health is a rather unexplored field. Among the possible applications, recent experimental studies[2] report that addition of nanoparticles (typically metal oxides nanopowders, such as Al2O3) can somehow modify the ignition mechanism of liquid fuels, probably influencing and accelerating the thermal exchange process between the fuel droplets and the surrounding air. In fact, the added nanoparticles can be small enough to approach molecular dimensions, modifying the physical properties of the liquid fuel (such as thermal conductivity, mass diffusivity and radiative heat transfer) and at the same time increasing the surface-area-to-volume ratio of the fuel droplets allowing more fuel to be in contact with the oxidant.These studies are focussed on the analysis of the ignition probability of additivated fuel single droplets impinging on a hot plate and it has been observed that ignition temperature of fuel mixtures containing nanoparticles is lower than that of pure Diesel fuel. In fact, ignition delay and ignition temperature are critical parameters to characterize the performance of a Diesel fuel, influencing both emission levels and efficiency of the combustion process. Moreover, it has been observed[3, 4] that the use of nanoparticles in rocket propulsion can enhance the engine performance under the point of view of specific thrust.Anyway, no data have been yet reported about the possible influence of nanoparticles addition upon the combustion features of a Diesel spray. Therefore, in this paper it has been experimentally analysed a stationary Diesel spray, produced by a gas assisted atomiser, preliminarily comparing the behaviour of the flame generated using the unseeded fuel with that one of the same fuel seeded with Al2O3 nanoparticles.
2. Experimental Set-up
- As previously outlined, the core of the apparatus consists of a gas assisted atomiser consisting of a capillary tube (0.3 mm inner diameter, 0.8 mm outer diameter) that lies in an opening of 1 mm diameter at the spray top, thus creating an annular gap. The liquid fuel flows under the action of a syringe pump (New Era Pump Systems Inc., Model NE-1000) inside the capillary at a flow rate of 1 ml/min. The dispersion gas (air in this application) flows inside the annular gap. The capillary ends a little downstream the opening, giving rise to an atmospheric spray flame ignited by a premixed methane-air flame which is concentrically arranged around the nozzle exit. This sustaining flame was kept just slightly rich, thus originating an overventilated cone in the presence of the oxidant. The gas flow rates are measured and controlled by thermal mass flow meters (Bronkhorst High Tech. The Netherlands). The apparatus is designed in order to achieve, through the longitudinal displacement of the dispersion gas injector, a variation of the outflow area, from zero to the maximum value. The combustion process has been confined inside a cylindrical quartz chamber (200 mm diameter), allowing full optical access, and the burned gases are conveyed in a hood connected with a sucking pump. The hood is equipped with a fibreglass filter (Whatman GF/A) placed rather far away from the flame tip, thus trapping the nanoparticles without been affected by hot gases.A schematic view of the whole apparatus is reported in Fig. 1.An easy way to comply with the paper
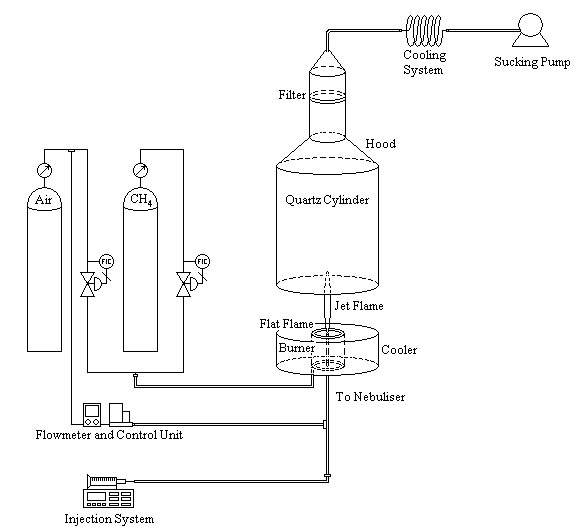 | Figure 1. The experimental set-up |
|
3. Results and Discussion
- The experimental analysis has been performed upon the stationary spray, operated at atmospheric pressure.In order to investigate the dimensional distribution of the generated droplets as a function of dispersion gas flow rate and gas to liquid mass ratio, the behaviour of the spray jet has been initially characterised in isothermal (cold) conditions, both through visualizations and PDA (Phase Doppler Anemometry). Figure 2 summarizes the most representative obtained results. Particularly, Fig. 2A shows the comparison of the counter mean diameter (CMD) for the water spray and the n-hexane spray at 5 mm from the burner exit by varying the oxidant flow. For flows of oxidant higher than 2 l/min the size of the droplet is almost constant, particularly for n-hexane, which behaviour can be considered representative of hydrocarbon fuel. N-hexane fuel produces smaller droplets than water (about 11 μm against 19 μm). The radial profile of CMD was also investigated. As an example Fig. 2B shows the CMD radial profile for the water spray at 50 mm from the exit nozzle. The diameter of the droplet is almost constant with a value of about 13 μm. It can be concluded that the “cold” spray is formed by droplets of about 15-20 m average size, with operating conditions almost constant inside a wide range of atomising air flow rate.After the preliminary characterization of the spray behaviour, a qualitative imaging of the generated flame has been performed varying the operating conditions (i.e. mainly the air flow rate). Fig. 3 reports images of the spray flame obtained burning the pure fuel at a fixed flow rate (input thermal power of the spray flame=700 W) varying the atomising air flow rate. It can be observed that a decrease of the air flow rate induces a progressive formation of intense yellow coloured combustion regions due to higher particulate formation from unburned species.
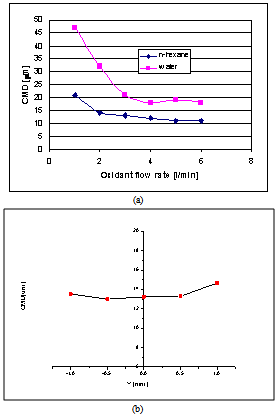 | Figure 2. A comparison of the counter mean diameter (CMD) for water and n-hexane spray at 5 mm from the exit nozzle - B) radial profile of the CMD for the water spray at 50 mm from the exit nozzle |
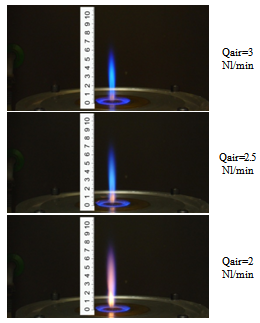 | Figure 3. images of the spray flame as a function of atomising air flow rate |
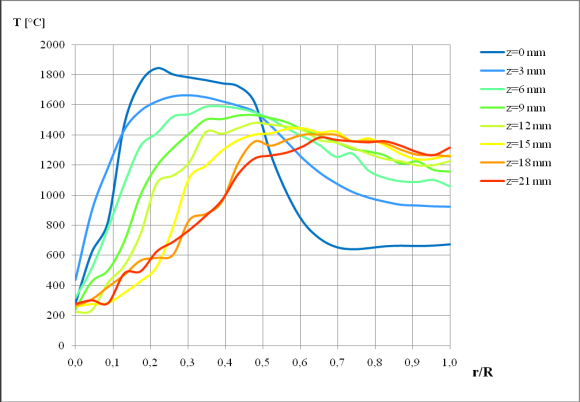 | Figure 4. Radial temperature profiles of the premixed flame |
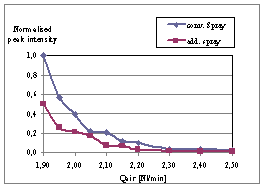 | Figure 5. Comparison of flame emission (band-pass filter at 597±30 nm) |
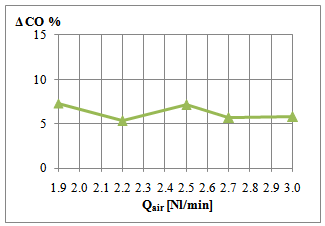 | Figure 6. Comparison of CO emission in the burned gases |
4. Conclusions
- The preliminary work performed suggests that the addition of nanoparticles to Diesel fuel (also at low concentration, i.e. 0.1% vol) can improve the combustion features of the spray flame, giving rise to lower CO emission levels and simulating a sort of higher-ventilation of the flame with respect to the unseeded spray, at identical air feeding conditions. Future experimental work is foreseen in order to deepen the results emerged till now, especially for sensitivity analysis of the spray flame to nanoparticles concentration in the fuel, typology and characteristic dimension of nanoadditives used, as a function of the operating conditions of the experimental set-up. Moreover, a theoretical study has to be performed in order to understand the influence of nanoparticles addition upon the combustion process, reaching a model of the involved phenomena.
ACKNOWLEDGEMENTS
- The Author is grateful to Dr. Francesco Cignoli and Dr. Giorgio Zizak for the help provided during the experimental measurements performed at CNR-IENI Institute in Milano, Italy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML