-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 22163-257X e-ISSN: 2163-2588
2012; 2(4): 104-108
doi: 10.5923/j.nn.20120204.03
Effect of Temperature on the Synthesis of Nano-Nickel Particles
Haque K M A 1, Hussain M S 2, Alam S. S 1, Islam S. M. S 1
1Shahjalal University of Science and Technology, Sylhet-3114, Bangladesh
2SIPCHEM, PO Box 130, Al-Khobar 31952, Kingdom of Saudi Arabia
Correspondence to: Haque K M A , Shahjalal University of Science and Technology, Sylhet-3114, Bangladesh.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The focus of this study is to observe the effect of temperature on the size and morphology of nano-nickel particles. Nano-nickel particles have been prepared by a simple polymer-surfactant interaction of a cationic polymer poly vinyl pyrrolidone (PVP) with an anionic surfactant, sodium-dodecyl-sulphate SDS at different temperatures i.e. 60℃, 80℃ and 100℃. Nano-sized nickel particles were synthesized by using nickel chloride as the precursor, hydrazine as the reducing agent in the presence of SDS and PVP in a strong basic medium (pH-10.2). Our research shows that the rate of reduction increases as the reaction temperature was increased from 60℃ to 100℃. Finer particles of diameters less than 10nm were formed as the temperature was increased from 60 to 100℃. The morphology of the synthesized nano-nickel particles also varied as the temperature was increased. The concentration of nickel chloride and SDS/PVP was kept constant in all these experiments. The nano-sized nickel particles synthesized were characterized by using SEM and HRTEM.
Keywords: Morphology, Hydrazine, Reduction, SEM, TEM
Article Outline
1. Introduction
- In the past two decades, considerable attention has been devoted to the synthesis of metallic nano-particles specially nano-nickel particles because of their unusual properties that differ from either the bulk or single atoms. During the 19th century and the first half of the 20th century the preoccupation with metal colloids was essentially confined within the academic communities as these materials offered excellent models for studying the relationships between the size, shapes and structure of the individual particles and their unique properties, such as , catalytic, optical, electrical and magnetic. During the second part of the last century, however, many of these properties have found applications in several fields of technology and as a result metallic particles ranging in size from few nanometres to ten of micrometers are today used extensively in electronics[1-4], catalysis[5-8], pigments[9] and metallurgy[10]. Several others applications such as bio-sensing[11-14], antimicrobial, non linear optics[15], high density magnetic storage[16], transparent conductive coatings[17] etc are still in early stages of development, but are expected to evolve rapidly within the next few years. More so than the traditional applications, these new technological fields require materials with controlled size, size distribution, morphology, composition, internal structure and surface characteristics. Consequentlythere is a renewed interest in the development of preparation techniques capable of tailoring these properties needs to support the technical progress of the existing applications and meet the challenges of the emerging ones.There are three different processes used for the synthesis of metallic nano-particles[18,19]. First process is “phase breakdown” in which by supplying energy necessary to increase the specific surface area of the dispersed matter, the size of the bulk metal can be reduced to the desired dimensions. This can be done by the mechanical breakdown of the solid metals using ball milling or by dividing the molten metal in to droplets, which are subsequently solidified by rapid cooling. These two methods are not capable of generating uniform or mono dispersed metallic particles. Second process is “phase transformation method” in which metal compounds are converted into finely divided metallic particles by thermal decomposition (thermolysis or pyrolysis) or chemical reduction. By this process it is possible to prepare uniform metal particles of different shape. Third process is “phase build-up”, in this process metallic particles are constructed from their building blocks i.e. metal atoms. This may be in a gas phase i.e. chemical or physical vapour deposition or in a liquid phase i.e. chemical precipitation. Different techniques have been used to prepare the metal nano-particles, such as reduction of metal oxides and metal salts[20], sonochemical and thermal decomposition of metal complexes[21] and reduction in solution by strong reducing agents[22].We report here synthesis of nano-nickel particles by a phase transformation method in which NiCl2 is converted to nano sized Ni particles through a chemical reduction process using hydrazine as a reducing agent along with a cationic polymer and an anionic surfactant in a basic medium. Surfactants-covered water pools offer a unique microenvironment for the formation of nano-particles. They do not only act as micro reactors for the processing reactions but also inhibit the excess aggregation of the particles because the surfactants could adsorb on the surfaces of the particles when the particle size approaches that of water pool. As a result, the particles obtained in such a medium are generally very fine and monodispersed.
2. Experimental
- Materials used for the synthesis of nano-nickel particles were nickel chloride analytical grade, sodium dodecyl sulphate, poly vinyl pyrrolidone, hydrazine hydrate solution sodium carbonate; de-ionized distilled water was used in the preparation of all the solutions. Instruments used for the preparation and characterization of nano-nickel particles were thermostatically controlled hot plate with magnetic stirrer, four necked round bottom flask, Condenser.Nano-sized agglomerated Ni particles were synthesized by dissolving 8.78g of NiCl2 (40 mmol) in a glass beaker containing 100ml of de-ionized distilled water at 40℃. The pH of the solution was maintained at 10.2 by adding Na2CO3 solution to the reacting solution. At this point the temperature of the solution was raised to 60℃ and 30 mL of hydrazine was added to the solution. In order to study the effect of temperature on the formation of nano-nickel particles, same experiment was carried out at 80℃ and 100℃.We observe that at 60℃ as the reaction continues, the appearance of gray/black precipitates in the beaker meant that nickel particles have started to form. This reaction is not instantaneous and can take several hours for the nickel ion reduction to reach completion. The particles from bottom of the reaction vessel were collected, centrifuged (4000 rpm), washed with distilled water and ethanol for three times, and finally desiccated at room temperature before characterization. Scanning Electron Microscope (SEM) FEI–NOVA 200Nanolab with EDAX and High Resolution Transmission Electron Microscope (HRTEM) JEOL - JEM 2100F had been used to characterize these synthesized nickel particles and elucidated results are discussed.
3. Results and Discussion
- Metal atoms are formed by transferring of electrons from a reducing agent (Redm-) to the oxidized metallic species Mn+
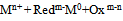 | (1) |
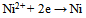 | (2) |
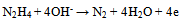 | (3) |
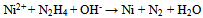 | (4) |
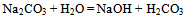 | (5) |
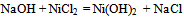 | (6) |
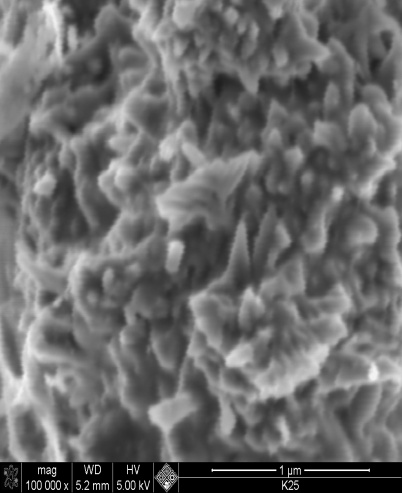 | Figure 1. SEM image nano nickel particles synthesized at 60℃, mag x100,000 |
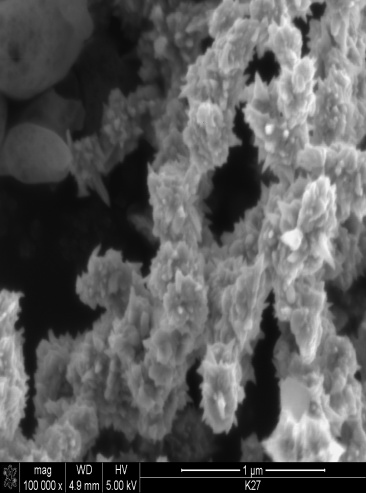 | Figure 2. SEM image of nano nickel particles synthesized at 80℃ mag x 80,000 |
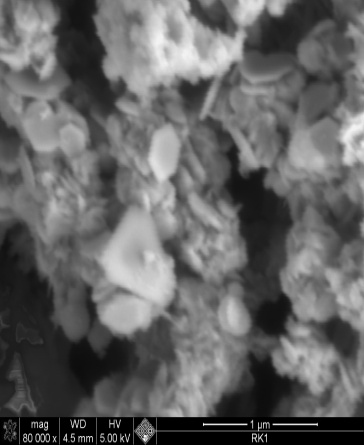 | Figure 3. SEM image of nano nickel particles synthesized at 100℃ mag x80,000 |
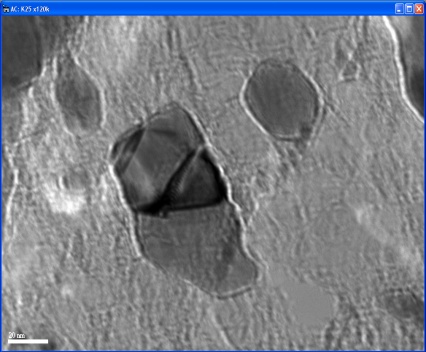 | Figure 4. TEM image of nano-nickel particles synthesized at 60℃ |
 | Figure 5. TEM image of nano-Ni particles synthesized at 80℃ |
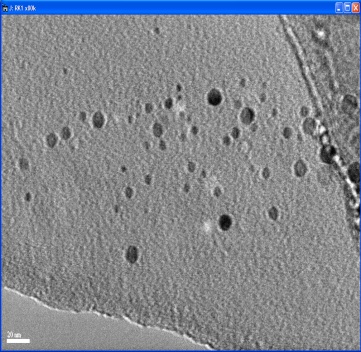 | Figure 6. TEM image of nano-Ni particles synthesized at 100℃ |
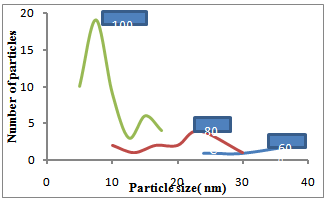 | Figure 7. Plot of Particle size versus number of particles at different temperature |
|
4. Conclusions
- Nano-sized nickel particles have been prepared by using a “phase transformation” method by using a chemical reduction process. In order to study the effect of temperature on the particle size, experiments were carried out at different temperatures i.e. 60, 80 and 100℃. SEM characterization showed that Ni particles synthesized at lower temperatures were almost spherical but as the temperature was increased particles showed very spiky morphology. However, finest Ni particles of less than 10nm in diameter were formed at 1000C. TEM characterization confirmed this inverse relationship i.e. Ni particle size decreased as the reaction temperatures were increased from 60 to 1000C. The focus of this study is in the applications of nano-Ni particles in aerospace and medical/biological applications.
ACKNOWLEDGMENTS
- The authors would like to thank the financial support received from King Abdul Aziz City for Science and Technology (KACST), Riyadh, Saudi Arabia under Project No. 26-05.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML