-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2012; 2(2): 14-21
doi:10.5923/j.nn.20120202.04
Gold Nanoparticles Conjugated Antibiotics: Stability and Functional Evaluation
Debalina Bhattacharya1, Biswarup Saha1, Ananda Mukherjee1, Chitta Ranjan Santra2, Parimal Karmakar1
1Department of Life Science and Biotechnology, Jadavpur University, Kolkata, WB, 700032, India
2Department of Chemistry, Netaji Nagar Day College, NSC Bose Road, Regent Estate, Kolkata, WB, 700092, India
Correspondence to: Parimal Karmakar, Department of Life Science and Biotechnology, Jadavpur University, Kolkata, WB, 700032, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Utilizing the combined reducing property of antibiotics and sodium borohydride, gold nanoparticles (Gnps) were co-synthesized with the antibiotics (ampicillin, streptomycin and kanamycin) and during this process antibiotics were conjugated with Gnps. The conjugation of nanoparticles was confirmed by dynamic light scattering (DLS) and electron microscopic (EM) studies. Such Gnps conjugated antibiotics when applied to three bacterial strains e.g. Escherichia coli DH5α, Micrococcus luteus and Staphylococcus aureus, showed greater bactericidal activity and reduced minimal inhibitory concentration (MIC) compared to their respective free forms. More significantly, all the three conjugated antibiotics displayed greater stability in UV, heat and prolonged storage at room temperature compared to their free form. Thus, our findings indicate that Gnps conjugated antibiotics are more efficient and may have significant therapeutic implications in the near future.
Keywords: Gold Nanoparticles, Antibiotics, Stability, Biological Activity
Cite this paper: Debalina Bhattacharya, Biswarup Saha, Ananda Mukherjee, Chitta Ranjan Santra, Parimal Karmakar, Gold Nanoparticles Conjugated Antibiotics: Stability and Functional Evaluation, Nanoscience and Nanotechnology, Vol. 2 No. 2, 2012, pp. 14-21. doi: 10.5923/j.nn.20120202.04.
1. Introduction
- Nanotechnology is the newly immerging field of studying the matter on nanoscale range, usually 100 nanometers or smaller in size (1, 2, 3). For the ability to deals with Nanoscale structures, nanotechnology has the potential to use as drug delivery agents or vectors (4, 5, 6), biosensors studies (7, 8) and improvement of the diagnosis of cancer and other diseases. Among all the nanoparticles gold nanoparticles provide a higher efficiency of light absorption at their longitudinal plasmon resonance. In particular, the enhanced absorption and scattering of gold nanoshells and nanosphere aggregates have been investigated for thermal therapy and biological imaging as they are less toxic to biological system (9). GNPs conjugated drug delivery was also drawn attention for their greater efficacy, delivery and lees toxicity. For example, Pinto-Alphandary et.al., reported that GNPs used as a potential carriers for ampicillin, as it allow increased drug deliberation at infected sites induced by Listeria, as well as decrease toxicity (10). It was observed that bacterial count in the liver was reduced at least 20 fold by the use of GNPs conjugated ciprafloxicin than the free drugs (11). For successful application of nano-antibiotic conjugates, apart from better delivery, their efficiency should be evaluated properly. Usually for the therapeutic purposes, the amount of antibiotics often given for the treatment is much higher than the dose required for killing the pathogens (12). This in turn could produce toxic effects, which was demonstrated in several reports too (13). For a successful antibiotic therapy, the dose should be reduced to avoid their side effects (14) Nanoparticle, specifically GNPs conjugated antibiotics may thus be used to address the problem associated with conventional antibiotic treatment. In such attempt it has been reported that ampicillin loaded nano particles used for the treatment of salmonella infection in mouse, reduced the drug by 40 folds compared to free ampicillin (15).Several strategies have been employed to conjugate antibiotics efficiently with nanoparticles especially with GNPs. In most of the cases, the conjugation was done by functionalizing gold particles, where amino acids, glutathione, polyethylene glycol etc were used as linkers (16,17). But some times addition of linker may either reduce the efficacy of the antibiotics or may also interfere with the stability of drugs (18). But to avoid the possible effects of these functionalizing agents on biological system, we have used a novel strategy where conjugations of the antibiotics were made during the synthesis of GNPs without using any linker (19). In our previous report, we have already compared the biological efficiency of GNP conjugated antibiotics with respect to their free forms in vitro (20). In present study, our main focus is to evaluate the stability of the AuNPs conjugated antibiotics compared to their respective free form. Three antibiotics ampicillin, streptomycin and kanamycin conjugated with GNPs were tested on three different bacterial system Escherichia coli DH5a (Microbial type culture collection (MTCC) No.1652, India), Micrococcus luteus (MTCC No. 106) and Staphylococcus aureus (MTCC No. 96) for their efficiency. Further, their stability was tested by keeping them long time in room temperature, or heat treatment, or UV treatment. In all these cases their efficiency was better than their respective free forms. Thus, these studies have a tremendous implication in the country like India, where storage of medicine in rural areas is a big problem.
2. Methods
- Synthesis of GNPsGold nanoparticles were synthesized by the standard chemical reduction method of chloroauric acid H(AuCl4) by sodium borohydride (NaBH4) at room temperature (19). A red wine colored suspension of GNPs was developed .This suspension was centrifuged, pellet washed three times & resuspended in deionized water so that excess ions may be removed. The suspension was sonicated before use. .Synthesis of conjugated GNPs with antibioticsAntibiotic conjugated gold nanoparticles were synthesized by combined reducing property of sodium borohydride & the three antibiotics i.e. Ampicillin, Kanamycin, Streptomycin respectively. A bluish colored suspension was developed after conjugation. Dynamic Light ScatteringThe Nano-ZS (Malvern) instrument (5 mW HeNe laserk = 632 nm) was used for this purpose. The sample was taken in a DTS0112—low volume disposable sizing cuvette of 1.5 ml volume (path length 1 cm). The operating procedure was programmed (using the DTS software supplied with the instrument) such that there were average of 25 runs, each run being averaged for15 sec, with an equilibration time of 3 min at 25℃. A particular hydrodynamic diameter (dh) was evaluated several times and distribution of dh was determined. Transmission Electron MicroscopyAll the three Gnps conjugated antibiotics along with the bare Gnps were prepared after drying on carbon coated copper grid and observed under a transmission electron microscope (FEI, Model: STWIN) with an accelerating potential of 200 KV and analyzed with TECNAI G2 software.Scanning Electron Microscopy GNPs conjugated antibiotics along with the bare GNPs after lyophilized on glass slides & coated with gold observed under a scanning electron microscope (JEOL JSM 5200).Bactericidal Activity MeasurementThis assay was conducted by standard agar well diffusion method. The E. coli DH5a, M. luteus and S. aureus strains were grown on LB Broth at 37℃ overnight upto a turbidity of 0.5 Mac Farland standard (108 CFU per ml) (21). About100 µl of this suspension was used to inoculate 90 mm diameter petridish filled with 30 ml of LB agar. Wells (diameter2 = 0.563 cm2) were punched in the agar plates and filled with 100 µl of either antibiotics or their respective GNPs conjugated forms. The concentrations of both the forms of antibiotics were at their respective MIC values, generally used in common laboratory purpose (50 µg/ml for ampicillin, 10 µg/ml for streptomycin and 50 µg/ml for kanamycin) (22). Plates were incubated at 37℃ for overnight. Antibacterial activities were evaluated by measuring the area of zone of inhibition (diameter2). We used autoclaved water and only GNPs as negative control.MIC Study of Free and Gnps Conjugated AntibioticsMIC of ampicillin, streptomycin and kanamycin along with their respective GNPs conjugated forms against E.coli DH5α, M. luteus and S. aureus in Luria-Bertani (LB) broth were determined by standard method[23]. Each tube contained 5 ml of LB medium inoculated with 106 bacteria/ml. Decreasing concentrations of each antibiotic and their corresponding GNPs conjugated form were added to the respective tubes. After overnight incubation at 37℃ OD of each tube was measured at 600 nm using a double beam spectrophotometer.
3. Results
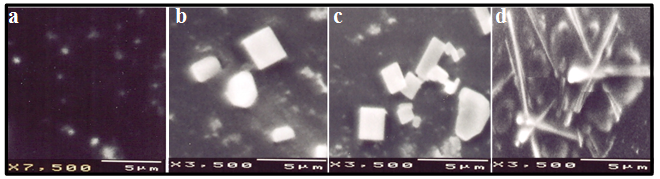
- Gold nanoparticles can be synthesized by using citrate or borohydride as the reducing agents. Here we have used sodium borohydride as a reducing agent because use of citrate needs continuous heating, which may interfere with the stability of the antibiotics before conjugation. We have used the ratio of the reductant and reducing agents in such a way that synthesized GNPs were less than 14 nm in size as determined by Dynamic Light Scattering (data not shown). The Surface Plasmon resonance of free GNPs was obtained at 526 nm but when the antibiotics were conjugated with free gold nanoparticles larger particles were produced and the color turned from red wine to purple. Therefore a red shift was observed by antibiotics conjugated gold nanoparticles in the surface plasmon resonance experiment (data not shown). From the intensity distribution plot we found that bare GNPs were monodisperse in nature where as the conjugated forms showed polydispersity (data not shown) confirming the successful attachment of antibiotics with Gnps. We then wanted to visualize the morphology of such conjugated nanoparticles by electron microscopic study.In the transmission electron microscopy, we have observed that the GNPs conjugated antibiotics produce larger particles (Fig.1). The conjugation with antibiotics results an irregular but consistence change in the particles association for all the three antibiotics tested. But in Scanning Electron Microscopy (SEM) distinct structures were found for all three antibiotics conjugated GNPs.GNPs conjugated ampicillin showed cubic structure, GNPs conjugated streptomycin showed rectangular rod shaped structure and GNPs conjugated kanamycin showed extended star like structures (Fig.2). After characterizing the physical properties of the antibiotics conjugated GNPs we want to check their biological activity. So, we next determined the minimal inhibitory concentration (MIC) of free antibiotic compared to their GNPs conjugated form in all the three bacterial strain mentioned earlier. MIC for each of the GNPs conjugated antibiotic reduced significantly compared to their respective free forms (Fig3, 4 and 5).For GNPs conjugated ampicillin, the MIC value was 45
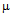 g/ml compared to 50
g/ml compared to 50 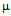 g/ml for free ampicillin (Fig. 3a), the corresponding values for streptomycin the corresponding values are 7 and 14
g/ml for free ampicillin (Fig. 3a), the corresponding values for streptomycin the corresponding values are 7 and 14 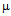 g/ml (Fig. 3b) and for kanamycin the values are 12 and 30
g/ml (Fig. 3b) and for kanamycin the values are 12 and 30 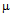 g/ml (Fig. 3c) in E. coli DH5α. In case of S.aureus the MIC value of GNPs conjugated ampicillin was 30
g/ml (Fig. 3c) in E. coli DH5α. In case of S.aureus the MIC value of GNPs conjugated ampicillin was 30 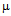 g/ml compared to 42
g/ml compared to 42 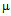 g/ml for free ampicillin (Fig. 4a) and for kanamycin the values were 5.5
g/ml for free ampicillin (Fig. 4a) and for kanamycin the values were 5.5 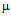 g/ml and 9
g/ml and 9 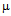 g/ml (Fig.4b). As S. aureus is streptomycin resistant so no MIC value was obtained. For M. luteus MIC value of AuNP conjugated ampicillin is 30
g/ml (Fig.4b). As S. aureus is streptomycin resistant so no MIC value was obtained. For M. luteus MIC value of AuNP conjugated ampicillin is 30 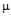 g/ml compared to 50
g/ml compared to 50 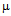 g/ml for free ampicillin(Fig. 5a), for streptomycin the values are 18
g/ml for free ampicillin(Fig. 5a), for streptomycin the values are 18 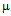 g/ml and 22
g/ml and 22 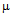 g/ml (Fig.5b) and for kanamycin MIC values were 25
g/ml (Fig.5b) and for kanamycin MIC values were 25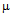 g/ml and 30
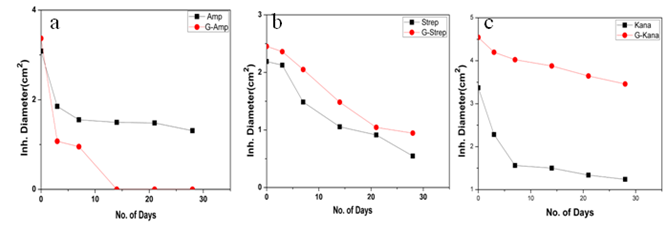
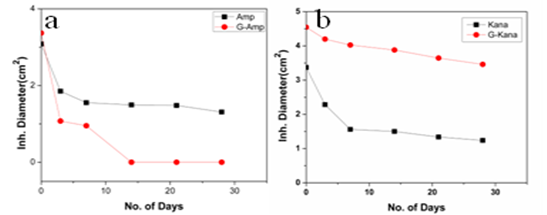
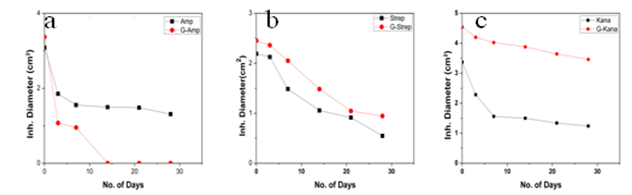
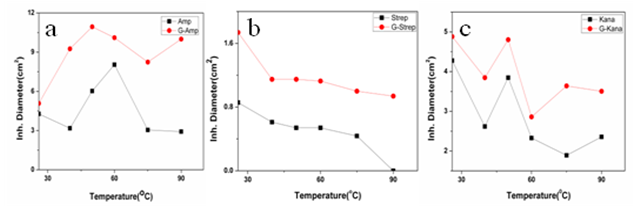
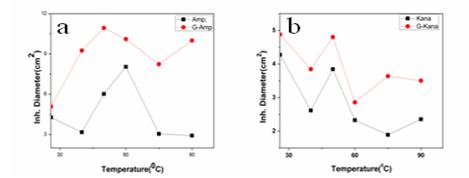
g/ml and 30 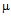 g/ml respectively(Fig. 5c). Thus in all the cases MIC values decreased significantly for GNP conjugated antibiotics compared to free antibiotics.We then observed the stability of the GNPs conjugated antibiotics compared to the free antibiotics. Both forms of the three antibiotics were stored at room temperature (25–28℃) for different time prior to assay and then their antibacterial activity was measured by agar well diffusion method.
g/ml respectively(Fig. 5c). Thus in all the cases MIC values decreased significantly for GNP conjugated antibiotics compared to free antibiotics.We then observed the stability of the GNPs conjugated antibiotics compared to the free antibiotics. Both forms of the three antibiotics were stored at room temperature (25–28℃) for different time prior to assay and then their antibacterial activity was measured by agar well diffusion method.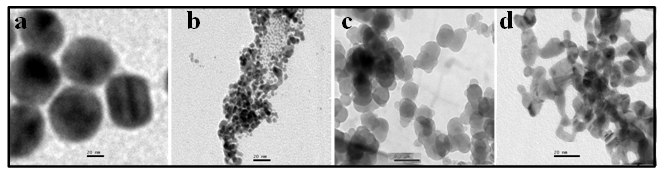 | Figure 1. Transmission electron micrographs of free GNPs and antibiotic conjugated GNPs. (a) Bare GNPs (b) Ampicillin conjugated GNPs (c) Streptomycin conjugated GNPs (d) Kanamycin conjugated GNPs |
 | Figure 2. Scanning electron micrographs of free GNPs & antibiotic conjugated GNPs. (a) Bare GNPs (b) Ampicillin conjugated GNPs (c) Streptomycin conjugated GNPs (d) Kanamycin conjugated GNPs |
4. Discussions
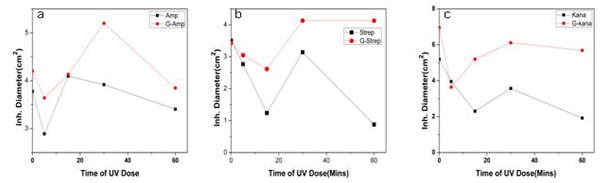
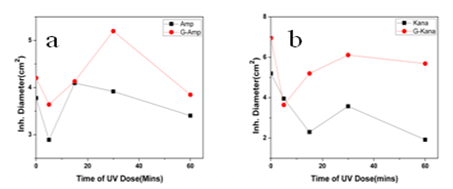
- In this work we have evaluated the efficacy as well as stability of GNP conjugated antibiotics ampicillin, streptomycin and kanamycin on three bacterial strains. Most of the nanoparticles are modified with functionalizing agents in order to conjugate various drugs for successful application in therapeutic purposes. The addition of functionalizing agents may sometimes interfere with biological system so biodegradable agents are now being considered. We have developed a unique system where addition of such agent can be avoided. The antibiotics attached with the GNPs have been proved by different physical techniques. Though the mode of interaction between GNP and antibiotics are not confirmed but it seems likely that antibiotics are adsorbed on the nanoparticles surface. The reducing property of antibiotics may be responsible for the adsorption. While addition of antibiotics to pre-synthesized nanoparticles did not shown any conjugation, clearly demonstrated that during reduction of auric chloride antibiotics are adsorbed. Moreover different antibiotics adsorbed on the nanoparticles surface in different manner resulting a specific structure, as observed in scanning electron microscopy. The resulting structure has more antibacterial activity and less MIC values. Among them the MIC values of ampicillin has been shown to less significant. This may be due to the fact that ampicillin precipitate faster from the suspension. This was also found while we studied the stability where GNP conjugated ampicillin is less stable than free ampicillin. The increment of the stability of the antibiotics may be due to the close association of antibiotics with GNP, which in turns increased the bond energy of the antibiotics. The conjugation of antibiotics may further enhance the concentration of the antibiotics inside the cells. This is may be achieved by better delivery of the antibiotics by nanoparticles. Another probability for enhanced activity may be due to the presence of strong reducing agent borohydride. But after preparation of the conjugated nanoparticles we have washed them repeatedly, so that presence of borohydride or other free charge is minimum. At the elevated temperature GNP conjugated ampicillin was shown to be more active. This was perhaps due to the presence of the delocalization of the electron in the carbonyl group of the β-lactam ring in ampicillin at elevated temperature. This in turn stabilized the GNP conjugated ampicillin. For GNPs conjugated streptomycin and kanamycin, heat stress induced more bond energy resulting increased in stability as well as activity. Moreover, introduction of energy by giving heat stress may thus increase the bond energy between GNPs and antibiotics giving unusual stability of the antibiotics. Similarly incubation in the presence of UV may increase the bond energy. Further, UV may also crosslink the antibiotics with GNPs resulting more stabilization of the later compared to free antibiotics. Thus in all the cases, the stability of the GNP conjugated antibiotics increased significantly. This is the most significant observation, particularly in Indian context, where proper storage facilities in remote area is not available.
ACKNOWLEDGEMENTS
- Debalina Bhattacharya is a junior research fellow of state govt. fellowship scheme, of Jadavpur University, West Bengal, India.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
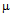 g/ml for ampicillin (a), 10
g/ml for ampicillin (a), 10 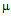 g/ml for streptomycin (b), 50
g/ml for streptomycin (b), 50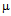 g/ml for kanamycin (c) and MIC for GNPs conjugated antibiotics is 45
g/ml for kanamycin (c) and MIC for GNPs conjugated antibiotics is 45 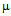 g/ml for ampicillin (a), 7
g/ml for ampicillin (a), 7  g/ml for streptomycin (b), 12
g/ml for streptomycin (b), 12 g/ml for kanamycin (c)
g/ml for kanamycin (c)
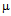 g/ml for ampicillin (a) 50
g/ml for ampicillin (a) 50 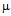 g/ml for kanamycin ( b) and MIC for GNPs conjugated antibiotics are 30
g/ml for kanamycin ( b) and MIC for GNPs conjugated antibiotics are 30 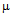 g/ml for ampicillin (a) 5.56
g/ml for ampicillin (a) 5.56 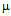 g/ml for kanamycin (b). S .aureus is streptomycin resistant
g/ml for kanamycin (b). S .aureus is streptomycin resistant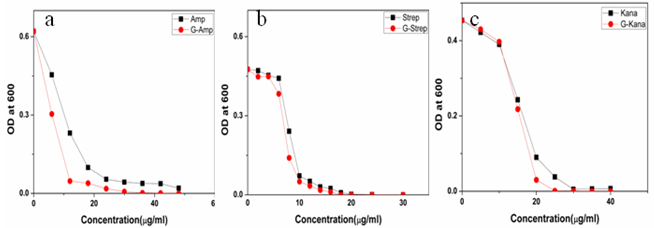
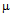 g/ml for ampicillin (a) 10
g/ml for ampicillin (a) 10 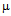 g/ml for streptomycin (b) 50
g/ml for streptomycin (b) 50 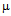 g/ml for kanamycin (c) and MIC for GNPs conjugated antibiotics are 30
g/ml for kanamycin (c) and MIC for GNPs conjugated antibiotics are 30 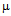 g/ml for ampicillin (a), 18
g/ml for ampicillin (a), 18 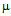 g/ml for streptomycin (b), 25
g/ml for streptomycin (b), 25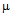 g/ml for kanamycin (c)
g/ml for kanamycin (c)