-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 22163-257X e-ISSN: 2163-2588
2012; 2(2): 5-8
doi: 10.5923/j.nn.20120202.02
Microwave Assisted Synthesis of Polyacrylonitrile/Ferrite Nanocomposites
T. Agarwal 1, K. Gupta 2, M. G. H. Zaidi 1, S. Alam 3
1Department of Chemistry, G.B.Pant University of Agriculture & Technology Pantnagar, Uttarakhand, 263145, India
2Department of Chemistry, Hindu PG College, Moradabad, 244001, India
3Polymer Division, Defense Materials Stores Research Development and Establishment, G.T. Road Kanpur, 208013, India
Correspondence to: M. G. H. Zaidi , Department of Chemistry, G.B.Pant University of Agriculture & Technology Pantnagar, Uttarakhand, 263145, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A microwave (MW) assisted green process to synthesize nanomagnetic polymer composites (NPCs) based on polyacrylonitrile (PAN) has been developed. The process involves 2,2-azobisisobutyronitrile initiated in-situ polymerization of acrylonitrile in presence of tween -20 stabilized ferrite nanoparticles (FNPs) under MW irradiation ranging 25 to 100 Watt over 10min. With MW power, the polymerization reaction has been progressed resulting in NPCs with enhanced rheoviscosity and yield over PAN. The formation of NPCs has been ascertained through Fourier transformed infra-red spectra, X ray diffraction and atomic force microscopy .Vibrating sample magnetometry and simultaneous thermogravimetric-differential thermal analysis-differential thermogravimetry reveals the formation of thermally stable NPCs with saturation magnetization 70.0 emu/g.
Keywords: Microwave Synthesis, Polymer Nanocomposites, Spectra, Microscopy, Magnetometry, Thermal Analysis
Article Outline
1. Introduction
- Nanomagnetic polymer composites (NPCs) based on polyacrylonitrile (PAN) and their copolymers offers wide spectrum of applications for dielectric ,microwave absorbing materials, data storage devices , sensor and biomedical applications[1-2]. Over past few years there is critical need for improved sustainability in the nanocomposite manufacturing through the development of green chemical approach. In this context, the significance of environmentally benign methods based on radiations and supercritical fluids as has recently been of increasing technological interest[3-4] .The physical characteristics of NPCs can be tailored through dispersing the FNPs into the polymer matrix either through reactive or non reactive routes in aqueous and organic phases. In the reactive process, the presence of FNPs induces acceleration of the free radical polymerization reactions under MW irradiation[5]. Reactive synthesis of NPCs has been conducted in presence of FNPs through in-situ polymerization[6].On the contrary, the non-reactive route involves the direct infusion of FNPs into the polymer matrix swollen in organic solvents. Literature survey reveals that NPCs based on PAN were synthesized through electrospinning of PAN in presence of FNPs in organic solvents[1,7-8]; no efforts are made to synthesize the proposed class of NPCs through MW assisted in-situ polymerization process.
2. Experimental
- Acrylonitrile (sd Fine, Chemicals, ) was purified through distillation under reduced pressure. AIBN () and other solvents were used without further purification. FNPs were prepared according to the method reported earlier[9]. FNPs (5.0 phr) were dispersed in T -20 (0.6mL) in a glass viol (10mL) under vortex over 25 min. The composition was further treated with AN (15.17X10-3 mol) and AIBN (0.61 X10-3 mol), followed by vortex for 2 min. The contents were subjected to MW irradiation at 25W over 10 min. The synthesized NPCs was isolated and washed with methanol. PAN was synthesized under identical reaction conditions in the absence of FNPs and used as a control.
2.1. Measurements
- FT-IR (KBr) spectra of samples were recorded on Galaxy 300 Mattson FT-IR spectrometer. Rheoviscosity were recoded at 27±1℃ over Nach Hoppler Viscometer at @ 1mg/mL in DMF.XRD pattern of samples was recorded at 25℃ over Rigaku-Geigerflex X-Ray diffractometer using Cu-Kα radiation (λ= 0.154056nm) in the 2θ range of 20º-70º at 40 KV and 20 mA with step size 0.019o. The crystallite size was evaluated through Debye-Scherrer equation. TEM images were recorded over JEOL 1011 at 80 kV. AFM images were recorded over NTEGRA Prima microscope under tapping mode using ultra sharp Si cantilevers having force constant of 48 N/m. VSM data were recorded over Princeton EG&G model 155 with maximum current 22.5 Ampere, scan time-900 seconds, VSM ranging 10 KOe to -10 K Oe at room temperature. Ferrite content of NPCs was deduced according to the relation % ferrite content =Ms of NPCs/Ms of FNPs X100, where Ms is saturation magnetization. Simultaneous TG-DTA-DTG was recorded over Perkin Elmer Pyris Diamond TA in air at the flow rate of 200mL/min up to 700℃/ 10℃/ min using alumina[9].
3. Result and Discussion
- MW assisted polymerization of AN monomer in presence of AIBN and a ferrofluid comprising FNPs (5.0 phr) stabilized into tween-20 has afforded Polyacrylonitrile ferrite nanocomposites (NPCs). The process of polymerization has been executed under MW irradiation ranging 25 to 100 W over 10min. In order to have results to be comparable, the NPCs was synthesized under the conditions similar to the polymerization reactions of AN. In general, with MW power, the polymerization of AN was progressed resulting in PAN with %Y ranging 13.65-9.93 and corresponding η (mPa.sX102) ranging 0.79-0.10With MW power, a regular decrease in η and %Y of PAN and respective NPCs has been observed. In general, such decrease in η and %Y has been higher in PAN over respective NPCs. Polymerization of AN in presence of FNPs (5 phr) was progressed over 10 min resulting in NPCs with η ranging 1.25-1.02. With MW power, polymerization of AN in presence of FNPs (50 mg) was progressed over 10 min resulting in NPCs with %Y ranging 71.66-21.55. Above 25W, the low η and %Y may be due to the MW assisted degradation of the polymers from the NPCs[10].
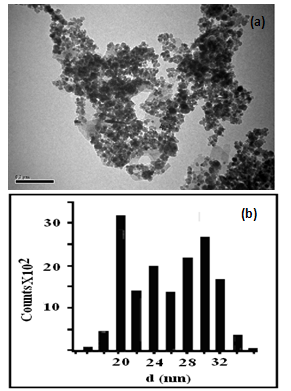 | Figure 1. Transmission electron micrograph (a) and particle size distribution (b) of FNPs |
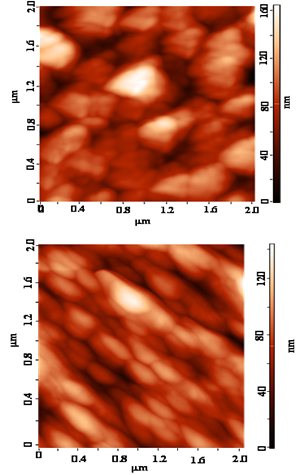 | Figure 2. AFM image of PAN (a) and NPCs (b) |
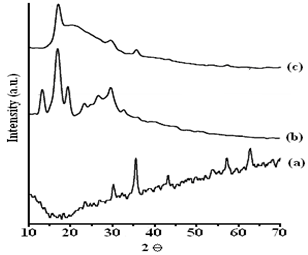 | Figure 3. XRD Spectra of FNPs (a), PAN (b) and NPCs (c) |
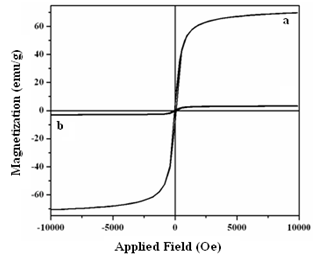 | Figure 4. Hysteresis curves of FNPs (a) and NPCs (b) |
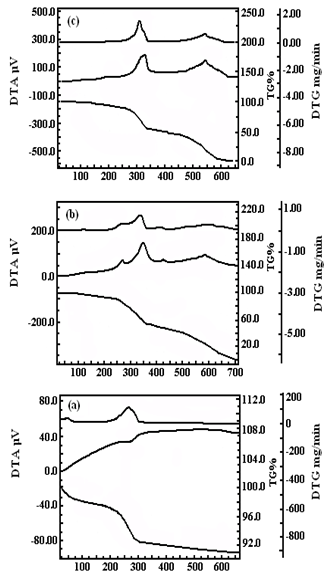 | Figure 5. TG-DTA-DTG of FNPs (a), PAN (b) and NPCs (c) |
4. Conclusions
- Present study describes the green synthesis of nanomagnetic polymer composites (NPCs) with enhanced thermal stability and saturation magnetization 70 emu/g through polymerization of acrylonitrile in T-20 stabilized ferrofluid under microwave irradiation ranging 25-100W. For this purpose ferrite nanoparticles (FNPs) with crystallite size ~18.20 nm were prepared using Fe (II) chloride as precursor. The formation of NPCs has been ascertained through spectra and microscopy.
ACKNOWLEDGEMENTS
- The financial assistance granted by Department of Biotechnology, Government of India is duly acknowledged. The Authors are also grateful to Instrumentation Center, IIT Roorkee, India.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML