-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 22163-257X e-ISSN: 2163-2588
2012; 2(2): 1-4
doi: 10.5923/j.nn.20120202.01
Study and Computational Simulation of Nanomaterial Coating to Reduce Crevice Corrosion
Jeremy Li
University of Bridgeport
Correspondence to: Jeremy Li , University of Bridgeport.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The crevice correction is a common type of localized corrosion which costs multibillion dollar losses in many different fields including aerospace, automobile, and building structures. The crevice geometry can easily catch moisture and impurity which accelerates the chemical corrosion. It is one of the dangerous corrosions that can cause sudden failure of the products and involve the human injury in some cases. Nanomaterial coating can disperse extremely tiny platelets into the crevice geometrical surfaces and make much strong and durable material coating to significantly reduce the crevice corrosion. This paper focuses on reducing the crevice corrosion damage by introducing the computer-aided modeling and simulation of nanomaterial coating on crevice structure to verify the nano-coating performance. The sample experiment to prove the strong anti-corrosion of nano-coating on crevice structure has also been performed to compare with the computational simulation results. Both results show that the nano-coating has much strong anti-corrosion performance over currently existing anti-corrosion coating techniques.
Keywords: Crevice Corrosion, Nano-Coating, Computational Simulation, Anti-Corrosion, Cost-Saving,Nanotechnology
Article Outline
1. Introduction
- Crevice corrosion is a localized corrosion that can happen in some good anti-corrosion materials including stainless steel[1]. This type of corrosion could not be easily to be detected at the early stage of the corrosion due to its invisible areas[2]. Because of this reason, sometime the products can have sudden malfunction and cause catastrophic failures which even can lead human injury[3]. The crevice geometry can easily catch and trap the moisture and chemical pollutes that speed up the corrosion process[4]. Crevice corrosion can happen in many locations including riveted, threaded, and welded areas. Nano-coating can be produced through dispersing the extremely tiny particles into the material surfaces to provide materials for strong anti-corrosion, durable material performance, and other super material physical properties[5,6]. The nanomaterial structure helps reducing the diffusive coefficient of liquids and gases to significantly trim down the corrosion process[7]. Researches on nanomaterial were advanced and promoted by the fact of considerable reduction of material corrosion by using nanotechnology.
2. Anti-corrosion Mechanism of Nanocoated Materials
- The anti-corrosion mechanism of nanocoated materials can be illustrated by referencing the Fig. 1[8].
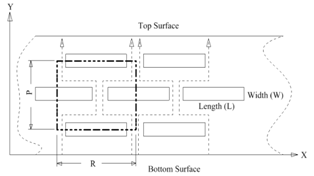 | Figure 1. Illustrated diagram for diffusive molecules through nanocoated material |
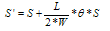 | (1) |
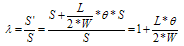 | (2) |
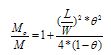 | (3) |
3. Computer-aided Modeling and Simulation of Nano-Coating
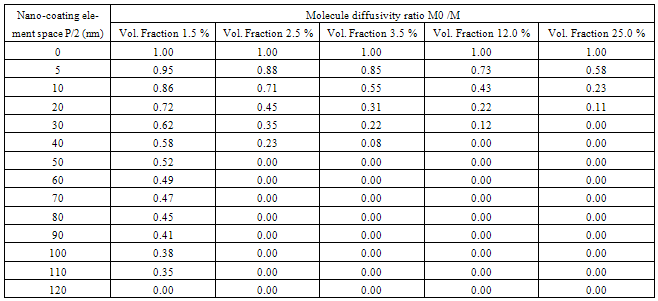
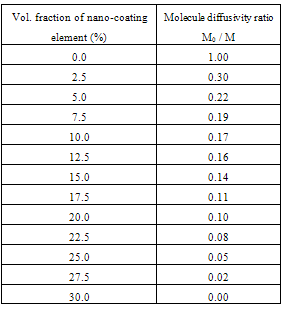
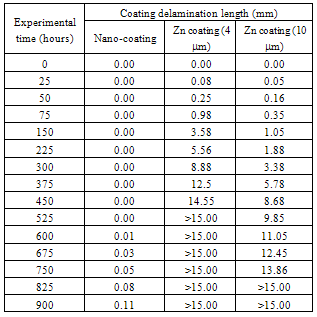
- The computer-aided modeling and simulation tool developed in this research can assist us to understand the mechanism of corrosion process and reduction of material corrosion through nanotechnology.Referencing Fig. 1, assume N0 and N the diffusive concentrations of molecules at the bottom and top surfaces for structural analysis (FEA) boundary conditions. The element dimension is P x R. Applying all these parameters into the computer-aided modeling, the simulation results are shown in Figs. 2, 3 and 4. The Fig. 2 depicts the relation between molecule diffusivity ratio of M0 / M vs. nano-coating element space P/2, under different volume fraction of nano-coating elements.Fig. 3 shows the result of molecule fraction ratio of Mo/M vs. volume fraction of nano-coating element.Fig. 4 indicates the relation between coating delamination and experimental time.
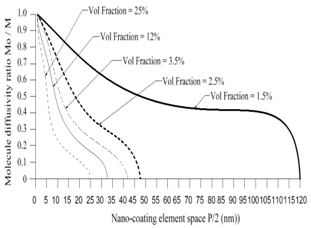 | Figure 2. Molecule diffusivity ratio of M0 / M vs. nano-coating element space P/2 |
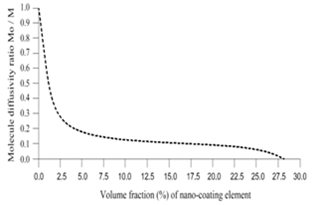 | Figure 3. Molecule diffusivity ratio of M0/M vs. volume fraction of nano-coating element |
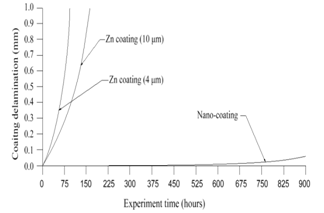 | Figure 4. Coating delamination vs. sample experiment time |
4. Sample Experiment
- Based on the testing standard of ASTM B117 and ASTM D1654, the sample tests have been performed to study and observe the performance of coatings subject to accelerated conditions in corrosion environment. The testing indicates significant improvement of anti-corrosion by using this nano-coating material which improves both stability of formed thin coating layer and kinetics of passive thin coating layer. The corrosion rate after applying this nano-coating is substantially reduced if compared with electro-galvanized coating. During testing, different grain sizes with 8-22 nm have been used in 5% sodium chloride solution. The smaller the grain size is, the larger the breakdown potential, passive current potential and aero current potential are. The dissolution (corrosion) rate is much lower in nano-coated material due to its enlarged breakdown potential and increased barrier to anodic dissolution from nano-coated material surfaces. Nano-coated material can be inspected for the physical and mechanical properties through X-ray diffraction technology and detected results show that the nano-coating deposits are produced in extremely small grain size. Material anti- corrosion performance relies on how efficient is the passive film developed, erosion extent of electrolyte, and property of electrode. In regular coating processes, the defect particles reside in the base metal materials, propagate through the coating film, and deposit in the grain boundary areas. In nano-coating process, the grain size is extremely small and grain number is significantly enlarged in which the defected particles are substantially diffused and evenly segregated which leads much stronger stress-resistant and corrosion- resistant coating films.The experiments have been performed on nano-coating and galvanized coatings to compare the results of computer-aided modeling and simulation. The testing results of molecule diffusivity ratio of M0 / M vs. nano-coating element space, molecule diffusivity ratio of M0/M vs. volume fraction of nano-coating element, and coating delamination vs. sample experiment time are shown in Tables 1, 2, and 3 respectively.The experimental results show that the molecule diffusivity ratio M0 / M will be decreased as volume fraction of nano-coating element is increased. Also the nano-coating has much superior anti-corrosion performance than the regular coating including Zn coating. All these experimental results are close to the computational simulation results introduced in this paper.
|
|
|
5. Conclusions
- This paper focuses on the study of mechanism of crevice corrosion and nano-coating performance based on computer-aided modeling and simulation to provide a fundamental understanding of anti-corrosion mechanism. The established computational model in this research can be used for further study and analysis on many different coating performances. The sampled crevice corrosions have been tested to verify the coating functionality. The computer- aided simulation result well matches the sample testing result and both results show that the corrosion rate in nanocoated products is significantly reduced compared with regular Zn coated products. Also, molecule diffusivity ratio M0 / M will be increased if the volume fraction of nano-coating element is decreased.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML