-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2012; 2(1): 16-21
doi:10.5923/j.nn.20120201.04
SiO2-TiO2 Nanostructure Films on Windshields Prepared by Sol-Gel Dip-Coating Technique for Self-Cleaning and Photocatalytic Applications
A. Shokuhfar1, M. Alzamani1, E. Eghdam1, M. Karimi2, S. Mastali3
1Mechanical Department (Advanced Materials and Nanotechnology Research Lab), K.N. Toosi University of Technology, Tehran, 19697 64499, Iran
2Metallurgy and Materials Engineering Department, Iran University of Science and Technology, Tehran, 13114 16846, Iran
3Faculty of Metallurgical and Materials Engineering, Semnan University, Tehran, 19111 35131, Iran
Correspondence to: M. Alzamani, Mechanical Department (Advanced Materials and Nanotechnology Research Lab), K.N. Toosi University of Technology, Tehran, 19697 64499, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In the present research, automotive windshield samples were successfully coated with SiO2-TiO2 nanostructure layer using the sol–gel technique for self- Cleaning and photocatalytic applications. This procedure resulted in transparent, crack-free, self-cleaning, nanostructure SiO2–TiO2 films. To prevent the thermal diffusion of the sodium ions from the glass substrate to TiO2 layer, the SiO2 layer was pre-coated on the glass by the sol–gel method. The coated samples were dried for 48 hour at room temperature to allow slow solvent evaporation and condensation reactions due to rapid sol–gel reaction of titania precursor. Then, the samples were annealed at 100℃ for 30 min and at the final temperature (500℃ and 700℃) for 30 min immediately. The crystalline structure, surface morphology, photocatalytic activity and hydrophilic properties of the films were investigated using XRD, SEM, FE-SEM, UV–Vis spectrophotometer and contact angle measurement, respectively. The FE-SEM surface morphology results indicate that the particle size increases from 19 to 42 nm by increasing the annealing temperature from 500℃ to 700℃. Likewise, XRD illustrate the crystal anatase and rutile as main phases for SiO2-TiO2 films annealed at 500℃ and 700℃, respectively. Increasing heat treatment temperature from 500 to 700℃, decreases the photocatalytic activity and inversely increases of the contact angle of the films.
Keywords: SiO2–TiO2, Nanostructure Layer, Self-Cleaning Films, Sol–Gel, Dip Coating, Windshield
Cite this paper: A. Shokuhfar, M. Alzamani, E. Eghdam, M. Karimi, S. Mastali, SiO2-TiO2 Nanostructure Films on Windshields Prepared by Sol-Gel Dip-Coating Technique for Self-Cleaning and Photocatalytic Applications, Nanoscience and Nanotechnology, Vol. 2 No. 1, 2012, pp. 16-21. doi: 10.5923/j.nn.20120201.04.
Article Outline
1. Introduction
- The development of automotive industries has always been paced by the results of research and development in the concurrent fields of design and materials technology. Production quality increase, such as self-cleaning glasses in windshields application, catalysts to reduce pollutants and etc, have led to more efficient and commodious manufacturing, and surface engineering is now a key materials technology in the design of future advanced automotive industries. Hereto, researches tend to TiO2 films as coating for windshields and lateral mirrors to benefit its self-cleaning advantages. Transparent SiO2-TiO2 films on glass could form the basis for self-cleaning of indoor windows, lamps or windshields.Since the past decades, an increasing interest has been devoted to the study of titanium dioxide (TiO2) thin films. TiO2 thin films have attracted considerable attention of use such as desensitized solar cells[1], photoelectrodes[1], photocatalysts[2,3], electrochromic displays[4], waveguides [5], gas sensors[6], resonators[7], and biomaterials[8], due to its high activity, photochemical inertness, non-toxicity, efficiency, and low cost.To maximize utilization of Titanium Dioxide for practical industrial applications, it is necessary to develop TiO2 film type, especially for photocatalytic applications. Titanium Dioxide occurs in three different crystalline Polymorphic forms: rutile (tetragonal), anatase (tetragonal) and brookite (orthorhombic). Among these, the anatase phase usually exhibits the best photocatalytic behaviour, while the rutile phase is the most stable phase. Photocatalysts may be used as a suspension in an aqueous solution or it may be immobilized on to a supporting substrate. The immobilization method is more convenient for practical use since the main problem in the usage of TiO2 suspended in an aqueous solution is the separation of TiO2 nanoparticles after the photocatalytic reaction[9]. The lower amount of the rutile phase of TiO2, the higher amount of the anatase phase of TiO2 and the better photocatalytic activity[10-12].A variety of techniques have been used for the preparation of TiO2 films including chemical vapour deposition[13,14] sol–gel[2-4,15] sputtering[6,16] and electron-beam evaporation (EBE)[17,18]. TiO2 film properties strongly depend on their microstructure that should be strictly controlled in order to obtain a tailored performance.Among all the possible techniques existing to elaborate TiO2 films, the sol–gel process is now widely used[19]. The sol–gel coating method is one of the promising methods, because the microstructure of the film is easily controlled with changing the solution composition and deposition condition. In addition, it provides uniform, porous TiO2 films with large specific surface area, which is favourable in achieving good photoactivity[20]. This method allows the modification of the structure and the microstructure of the deposited titanium dioxide playing on the sol composition, the coating parameters and the thermal treatment conditions and etc. Furthermore, the sol–gel process does not necessitate the use of expensive equipments as the case of vacuum deposition techniques[21]. The properties of the sol–gel TiO2 thin films are highly dependent on the structure (amorphous or crystallized), the thickness and the density of the deposited layers. These features are then mainly influenced by the sol composition, the sol viscosity, the withdrawal speed, when the dip coating process is used, the substrate nature and the sintering mode[22,23].The purpose of the present study is to experimentally assess the SiO2-TiO2 films for advanced windshields to benefit the self-cleaning applications. Specifically, annealing temperature as the key feature of the layer which affects performance, microstructure and the fundamental phase that must be considered, is addressed.
2. Experimental
2.1. Materials
- Titanium tetra isopropoxide1 (TTIP, 99 %, PANREAC) and Tetraethyl ortosilicate2 (TEOS, 98%, ACROS) were used as precursors. 2-propanol3 (99.7%, MERCK) and ethanol4 (96%, MRECK) served as solvents; hydrochloric acid (HCL, 37%, MERCK) was used as a catalyst. diethanolamine5 (DEA, 99.5%, MERCK) was used as stabilizing agent and a modulator was added into the TiO2 sols.
2.2. Preparation of Sols
- The procedures for the silica sol preparation and its characteristics were presented in detail elsewhere[24]. TiO2 thin films were prepared by the sol–gel dip coating technique, which is based on the hydrolysis of alkoxides in alcoholic solutions. In this study, DEA with DEA/TTIP molar ratio of 1:1 was employed .The procedure of preparation includes the dissolution of the mixture of 1:25 moles of 2-propanol as solvent and TTIP as precursor with the mixture of 1:1 mole of DEA and distilled water.
2.3. Preparation of Samples
- The automotive glasses were used as the substrates. The specimens in the form of slides with dimension of 100mm× 20mm×2mm were used as the substrate to support the SiO2-TiO2 films. At first, substrates were cleaned in an ultrasonic cleaner with de-ionized water and then with acetone. Subsequently the prepared specimens were heated at the temperature of 60℃ for 1h and immediately coated.
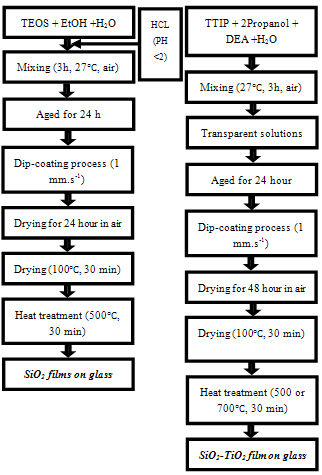 | Figure 1. Procedure used in this paper (left: preparation process of SiO2 films, right: the preparation process of SiO2-TiO2films) |
2.4. Dip Coating
- The substrates were dipped in the sol and withdrawn at a speed of 1 mm.s-1 to make a gel film. The coated films were dried for 48 hour at room temperature to allow slow solvent evaporation and condensation reactions due to rapid sol–gel reaction of titania precursor, then the samples were heated. The furnace temperature increased at heat rate of 10℃. min-1 until 100℃; hold at this temperature for 30 min. The coated substrates were dried at 100℃ for 30 min to improve the adhesion of films on glasses and to release residual stresses. The temperature of the furnace was subsequently increased at heat rate of 10℃. min-1 to the final temperature (500 and 700℃) and hold at this temperature for 30 min to accomplish the crystallization of gel films. Finally, the films were cooled in the furnace to room temperature. A flow chart of this method is presented in Figure. 1.
2.5. Characterization of TiO2 Films
- The main phase present was identified and the crystal size of the SiO2-TiO2 films was determined by X-ray diffractometer (XRD) (D8 Advance, Bruker Co., Germany) using monochromatic Cu–Kα radiation operated at 40 kV and 30 mA at the scan speed of 0.02 sec per step with an increment of 0.02° per step. The crystal size of the SiO2 - TiO2 films is calculated using the Scherrer’s equation based on the full peak width at half maximum intensity (FWHM).Film morphology was characterized by Scanning Electron Microscopy (SEM; Tescan model Vega-II) and Field- Emission-Scanning Electrons Microscopy (FE-SEM, S- 4160). Photocatalytic activity of the SiO2-TiO2 films was evaluated by investigating degradation of methylene blue (MB), which was used to represent a pollutant. For the photo degradation investigation, the SiO2-TiO2 films on glass substrates were immersed in an aqueous solution of MB (6.25×10-5 M) then irradiated with UV light for 2 h. After the radiation, degradation of the MB was determined by measuring absorbance of the MB of each decanted solution using UV–vis spectrophotometer (SERIES C-2040) at maximum wavelength of
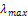 = 664 nm. The contact angle for water was determined using an OCA 15- plus (Dataphysics).
= 664 nm. The contact angle for water was determined using an OCA 15- plus (Dataphysics).3. Results and Discussion
3.1. Surface morphologies of SiO2–TiO2 films
- The surface morphology of the SiO2-TiO2 films were examined using SEM. Figure. 2 shows SEM micrographs of the SiO2-TiO2 films on substrate annealed at (A) 500℃ and (B) 700℃ for 30 min in air. Temperature increasing may lead to non-uniform and cracked coating and reduce coating lucidity and transparency (As can be seen in Figure. 2). This is due to substrate plasticity and softening at the temperature of 700℃.
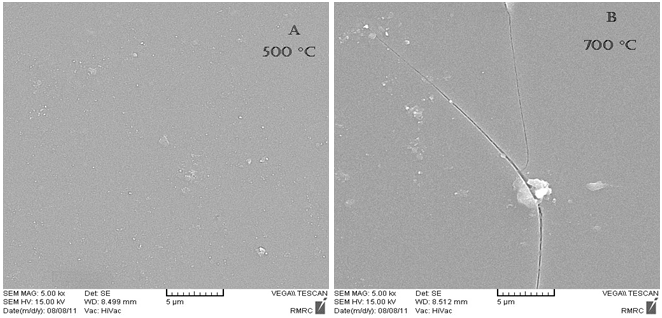 | Figure 2. SEM micrographs of SiO2-TiO2 films annealed at (A) 500℃ (B) 700℃ |
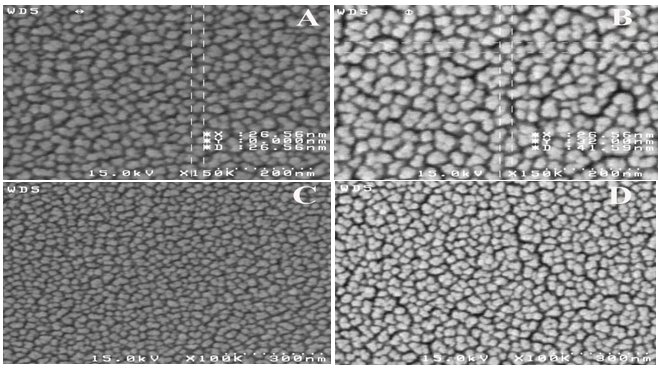 | Figure 3. FE-SEM micrograph of a SiO2-TiO2 films annealed at (A,C) 500℃, (B,D) 700℃ |
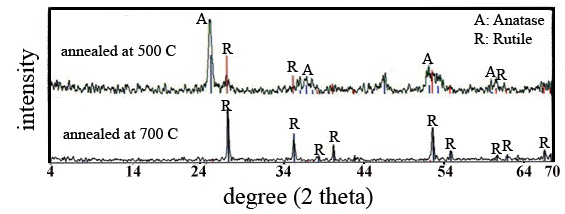 | Figure 4. XRD patterns of SiO2-TiO2 films produced on glass Substrates annealed at 500℃ and 700℃ for 30 min in air |
3.2. Crystal Structure of SiO2-TiO2 Films
- In order to compare the crystalline composition and crystal size of the films prepared at different annealing temperatures (500 and 700℃), XRD test was employed. Figure. 4 represents results of XRD analysis for the SiO2- TiO2 films.The XRD pattern clearly shows that the main peak of films annealed at 500℃ is at 25.28, which corresponds to anatase (101) plane. The main peak of Films annealed at 700℃ is at 27.44, which corresponds to rutile (110) plane. The peak corresponding to rutile phase can be clearly seen in the films annealed at 700℃ which has high intensity compared to prior sample. Relatively low annealing temperatures (for instance, 500℃ in this research) cannot lead to anatase-to-rutile crystal phase transformation. On the other hand, high annealing temperatures (for instance, 700℃ in this study) have led to the transformation of anatase to rutile. One can deduce from[25] that the anatase to rutile phase transformation take place at temperatures between 600-700℃. There is a decrease in amount of anatase phase in the crystalline plane of (101) after increasing annealing temperature from 500 to 700℃.The XRD results of the SiO2-TiO2 films at annealing temperature of 500℃ showed a phase distribution of 91:9 of anatase:rutile phases, with crystal size of 21 and 9 nm, for anatase and rutile, respectively. Also XRD results of the SiO2-TiO2 films at annealing temperature of 700℃ showed a phase distribution of 6:94 of the phase anatase:rutile, with crystal size of 52 nm for rutile. Results on average crystal size of the SiO2-TiO2 films at annealing temperature 700℃ of rutile phase (110) derived from XRD patterns revealed rutile as the main crystal phase, with a fundamental crystal size of 52 nm.
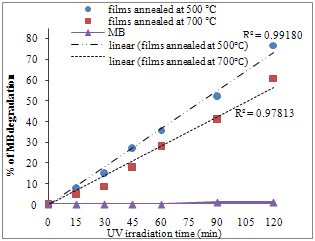 | Figure 5. A comparison of photocatalytic activities of the SiO2-TiO2 films annealed at (a) 500℃ (b) 700℃ |
3.3. Photocatalytic Activities of SiO2-TiO2 Films
- Figure. 5 shows the dependence of the MB concentration in the solution for the SiO2-TiO2 films annealed at 500℃ and 700℃ on the UV irradiation time. For both films, the concentration of MB in the solution decreases. As shown in Figure. 5, there was no direct photolysis of MB in the absence of the SiO2-TiO2 films. The SiO2-TiO2 films annealed at 500℃ exhibits higher degradation rate. This phenomenon seems to be due to the higher amount of the anatase phase of TiO2 and lower amount of rutile phase of TiO2. High hydrophilicity of coatings annealed at 500℃ compared to those annealed at 700℃ support this reason. The photocatalytic activity of the SiO2-TiO2 films annealed at 700℃ is lower being comparable with the SiO2-TiO2 films annealed at 500℃. The differences in photocatalytic activity could be contributed to the differences in phases which mainly exist in each films. The photocatalytic activity of the SiO2-TiO2 films shows a close dependence on heat treatment temperature.The photocatalytic degradation of the MB dye follows the Langmuir–Hinshelwood kinetics model given by the equation (1)[9]:
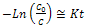 | (1) |
3.4. Surface Wettability of SiO2-TiO2 Films
- Surface wettability is a crucial factor for the self-cleaning of SiO2-TiO2 films when applied on windshields. A super hydrophilic surface favors the spread of water and the contaminant on the surface can be removed easily by the rainwater[26].Figure. 6 shows the contact angle of 4 µL water on the SiO2-TiO2 films. The contact angles less than 10° are recorded as zero, as the OCA 15- plus was not able to accurately measure contact angles below 10°.
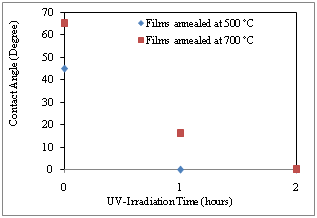 | Figure 6. Changes in the water contact angle of the SiO2-TiO2 films annealed at 500℃ and 700℃ (in a dark and after 1, 2 hours UV irradiation) |
4. Conclusions
- In the present research, SiO2–TiO2 nanostructure films were successfully coated on windshields using the sol–gel technique for self-cleaning applications. This paper presents the annealing temperature effect on the properties of the SiO2-TiO2 films. This research proved that annealing temperature is an important parameter which affect the structure; surface morphology, photocatalytic activity and surface wettability of the SiO2-TiO2 films.The FE-SEM surface morphology results indicate that the particle size increases from 19 to 42 nm by increasing the annealing temperature from 500℃ to 700℃. The SEM surface morphology results of the SiO2-TiO2 films annealed at 500℃ was uniformly and crack-free.XRD shows the crystal anatase and rutile as main phases for SiO2-TiO2 films annealed at 500℃ and 700℃, respectively. Films annealed at temperatures below 100℃ are not adhesive, do not adhere well to the glass substrate, and flake off into the solution. Annealing at 200℃ produces a film that can be removed from the surface by soft scratching. Films annealed at 500℃ are adherent. Annealing at 700℃ is not suitable and practical, because at this temperature glass substrate begins to soften and lucidity reduced. It was anticipated that too low temperatures would not eliminate the organic residues while too high temperatures could induce the transformation from anatase to rutile, which is in principle undesired for functional applications of the SiO2-TiO2 films.Increasing heat treatment temperature from 500℃ to 700℃ decreases the photocatalytic activity of the films. A clear diminution in the photocatalytic activity occurred at the temperature of 700℃. This can be assumed to result from the increasing conversion from anatase into rutile.In dark (without UV irradiation ), the SiO2-TiO2 films annealed at 500℃ with anatase phase are almost hydrophilic with the contact angle 45°, those annealed at 700℃ with rutile phase are relatively hydrophobic with the contact angle 65°. This change in contact angle is clearly due to an increase in the heat treatment temperature. After 1h UV irradiation, the wettabilities of the SiO2-TiO2 films become almost same. After 2 h UV irradiation, both films show super hydrophilic with contact angles less than 10°.
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge the support of the SAPCO CO. (SUPPLYING AUTMOTIVE PARTS CO.).
Notes
- 1. -(C12H28O4Ti) or Ti(OC3H7 i)42. -(C8H20O4Si) or Si(C2H5O)43. - CH3CH(OH)CH34. - C2H5OH5. - C4H11NO2
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML