-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2012; 2(1): 13-15
doi: 10.5923/j.nn.20120201.03
Computer-aided Modeling of Nanochrystalline Coating to Reduce the Galvanic Corrosion
Jeremy (Zheng) Li
University of Bridgeport
Correspondence to: Jeremy (Zheng) Li , University of Bridgeport.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The galvanic correction is a very common corrosion in today’s life, such as the corrosion produced in areas of welded joints and bolted fasteners. This corrosion tends to occur when different conducting materials are contacted electrically with expose to the electrolyte media. The different metals show different corrosion potentials when exposed to the electrolyte. Each year the corrosion issue causes not only the safety problems but also multiple billion dollars loss in many different fields including aerospace and automobile industries. This paper focuses on the fundamental study of galvanic corrosion mechanism, reducing the corrosion by introducing nanochrystalline coating, and improving the anti-corrosion mechanism design through computer-aided modeling and simulation. The computer modeling presented in this paper has been verified by comparing the testing results. Both computational and testing results are found close to each other which validate the creditability and feasibility of this anti-corrosion research.
Keywords: Corrosion Mechanism, Computer Modeling, Galvanic Corrosion Nano-Coating, Anti-Corrosion
Cite this paper: Jeremy (Zheng) Li , "Computer-aided Modeling of Nanochrystalline Coating to Reduce the Galvanic Corrosion", Nanoscience and Nanotechnology, Vol. 2 No. 1, 2012, pp. 13-15. doi: 10.5923/j.nn.20120201.03.
Article Outline
1. Introduction
- The galvanic current flow is driven by corrosion potential difference between the metal materials. Both the corrosion potential difference and galvanic flow resistance control the intensity of galvanic current flow[1]. The galvanic flow resistance includes resistance to the current flow in metals and bridge connection among metals, resistance related to the polarization of anode and cathode, and resistance to the current flow in electrolyte media[2]. To reduce the galvanic corrosion, the following items must be considered and controlled[3, 4]:1. Product geometrical conditions, such as geometrical shape in surface contact, surface area ratio in surface contact, and bridge distance in surface contact;2. Bridge conditions, such as connective methodologies of welding, bolting, and riveting;3. Metal composition;4. Metal galvanic potential in different alloy composition;5. Environmental factors, such as temperature and humidity; The geometrical area ratio is important to control the dissolution current density to the anode to reduce the galvanic corrosion. The galvanic current flow can be measured by zero resistance ammetry methodology[5]. Nano coating is currently an important corrosion resistan ttechnology due to its superior corrosion-preventive property[6]. Because of its extremely small grain size with its large grain boundary ratio, nanomaterial has superior material properties including excellent physical and mechanical functions[7]. Nanomaterial structures have a protective oxide scale that is strongly adhesive to the substrate of coated material with superior wear, scratch-resistant, and water-resistant properties[8, 9]. Nano coating combines outstanding strength and ductility. Environmental improvement can be achieved using nano coating technology compared with convenient coating technologies which can cause environment pollution[10].
2. Mechanism of Nanochrystalline Coating to Reduce Galvanic Corrosion
- Nanocrystalline coating material can be produced by methodology of electrodeposition which can be applied to the metals for corrosion protection. Control of grain size can improve wear resistance, hardness, mechanical and physical properties of nano coating material. The improved corrosion resistance for nanochrystalline coating has been evaluated through some techniques. The experiments indicates that the nanochrystalline coating of Fe38-Ni10-Cr16-P10-B10 with passivating alloy composition has superior corrosion resistance, compared with current coating techniques, due to increased Cr composition inside of coating film through enhanced internal material phase boundary diffusion. The research verifies that the imperfective intensity of grain boundary leads metal dissolution. If the Aluminum dissolves very fast inside of grain boundary, it can produce more stable passive thin layer and the oxide layer in nanochrystalline coating on metals can give much better corrosive protection. The further investigation shows that nanochrystalline coating material can be passive in lower acidic media where quick dissolution is detected. The enhanced grain boundary in nanochrystalline structure can compensate the loss of oxide compactiveness so that nanochrystalline coating material is more durable and reliable to prevent the corrosion in alkaline environment. The research demonstrates that the process of nanochrystalline catalyses the passive kinetics reduction and hydrogen reduction that leads more stable passive nanochrystalline thin layers, providing highly protective coating in fracture, wear and corrosion resistances.
3. Computer-aided Modeling and Simulation of Nanochrystalline Coating
- To establish the feasible and validated computational models to understand and analyze the anti-corrosion on various products and associated performance, this paper shows how computational modeling tools and testing methodologies assist us in developing a fundamental understanding of corrosion mechanism and in improving material anti-corrosion performance. The Fig. 1 shows the scratch resistance of nanochrystalline coating with different percentage of nanochrystalline material. The result indicates the super anti-corrosion property in nanochrystalline coating material.Fig. 2 compares the corrosion speed between galvanized and nanochrystalline coatings. The above result shows that the nanochrystalline coating has much less corrosion speed than galvanized coating due to the strong and enhanced corrosion resistance inside of nanochrystalline coating.The current density vs. potential of anodic polarization for galvanized coating and nanochrystalline coating is depicted in Fig. 3. Since the current density of galvanized coating changes more sharply than that of nanochrystalline coating, the nanochrystalline coating shows more stable, durable and positive performance.
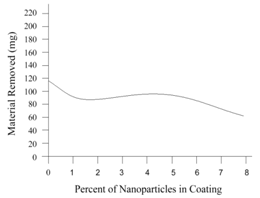 | Figure 1. Scratch resistance of nanochrystalline coating by computer- aided simulation |
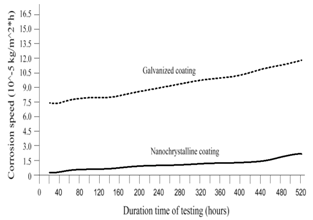 | Figure 2. Corrosion speed vs. time by computer-aided simulation |
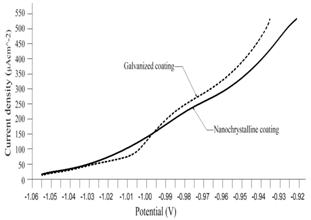 | Figure 3. Current density vs. potential of material anodic polarization |
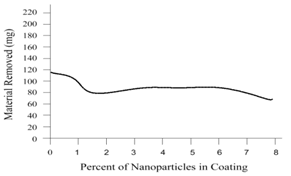 | Figure 4. Scratch resistance of nanochrystalline coating by sample testing |
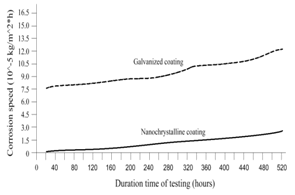 | Figure 5. Corrosion speed vs. time in 4.0 % NaCl solution by testing |
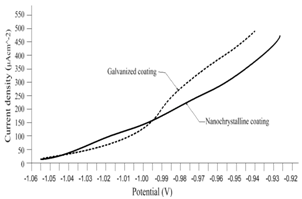 | Figure 6. Current density vs. potential of material anodic polarization by testing |
4. Conclusions
- This research introduces a validated computer-aided modeling methodology to simulate the galvanic corrosion and provides a fundamental understanding of anti-corrosion mechanism. This paper also establishes a computational simulation model to study and analyze the nanochrystalline coating performance. The galvanic corrosion samples have been tested to verify the computer-aided simulation model. Both computational modeling and sample testing demonstrate that the corrosion rate in nanochrystalline coating is much less than regular galvanic coating due to its strong corrosion resistance in nano-coating material.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML