-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2012; 2(1): 8-12
doi:10.5923/j.nn.20120201.02
Photo Catalytic Degradation of Chloropyrifos, Endosulphon, Imidocloprid and Quinolphos by Nano Crystalline TiO2 – a Kinetic Study with pH and Mass Effects
J. Santhanalakshmi1, R. Komalavalli2, P. Venkatesan1
1Department of Physical chemistry, University of Madras, Guindy Campus, A.C.Tech., Chennai, Tamil Nadu, 600025, India
2GKM College of Engineering and Technology, Chennai, Tamil Nadu, 600063, India
Correspondence to: J. Santhanalakshmi, Department of Physical chemistry, University of Madras, Guindy Campus, A.C.Tech., Chennai, Tamil Nadu, 600025, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Intense and massive agricultural methods ultimately lead to accumulation of pesticides in high concentrations for long durations of time in soil. With increasing demand for vegetables and agro products a crisis on the environmental problems and propagation of pollutants through food products prevail. Novel oxidation methods involving eco-benign, cheap and reusable catalysts are required for the degradation of pesticides. In the present work, among the widely used pesticides Chloropyrifos, Quinolphos, Imidocloprid and Endosulphon are chosen for degradation studies. Titanium dioxide (TiO2) is a popular photo catalyst, with emergence of catalysis by metal oxide nanoparticles, nano crystalline TiO2 was synthesized by adopting combination of hydrothermal and sol gel method has been utilized. XRD and SEM-EDX measurements are made for size characterization of nano TiO2 and it is found to be 20±0.5 nm. Under visible light absorbance variation with time is recorded and used for the determination of kinetic parameters such as rate coefficient. Effects of pH and catalyst mass are studied. The optimum pH values for maximum rates are found to be 4.8, 5.2, 5.8 and 6.5 corresponding to Chloropyrifos, Quinolphos, Imidocloprid and Endosulphon respectively. In the absence of TiO2 such reactions did not proceed even under visible light.
Keywords: Photo Catalytic Degradation, Nano Crystalline TiO2, Mass Effect, Ph Effect, Pesticides, Kinetic Study
Cite this paper: J. Santhanalakshmi, R. Komalavalli, P. Venkatesan, Photo Catalytic Degradation of Chloropyrifos, Endosulphon, Imidocloprid and Quinolphos by Nano Crystalline TiO2 – a Kinetic Study with pH and Mass Effects, Nanoscience and Nanotechnology, Vol. 2 No. 1, 2012, pp. 8-12. doi: 10.5923/j.nn.20120201.02.
Article Outline
1. Introduction
- Intense and large scale agricultural methods involve the use of a variety of pesticides (Ps), herbicides (Hs), insecticides (Is) and etc in massive concentrations with long time durations[1]. Most of such chemicals are resistant to degradation under natural environmental conditions[2]. In recent years there is a global concern on the adverse effects of cumulative presence of these Ps, Hs and Is in water, food and in air[3]. With increasing demand for vegetables and other agro products there exists a crisis on the environmental problems that develop due to slow oxidative degradation and mineralization of accumulated high concentrations of these Ps, Hs and Is. Hence novel oxidation methods involving advanced materials for the easy eco-benign, cheap, reusable and faster degradation favoring catalysts are wanted[4]. Currently UV-vis and photosensitive catalyst such as TiO2 which is popularly and extensively used in research work involving photo degradation of various organic compounds and on the family of Ps, Hs and Is, has been considered.With the advent of nano forms of the bulk materials particularly catalysts which perform efficiently than the bulk counterparts, the use of nano crystalline TiO2 for the purpose of catalyzing the degradations of Ps, Hs and Is in aqueous medium is a much needed process[5]. Smaller the particle size of the catalyst, especially in the nano meters, better the photo catalytic activity and has been attributed to the high surface area and surface area to volume ratio values[6].We have focused our study on the oxidative degradations of a four well known and widely used insecticides and pesticides such as chloropyrifos(Ch), Endosulphon(En), Imidocloprid(Im) and Quinolphos(Qn) in aqueous medium in the presence of nano crystalline TiO2 as synthesized and characterized in the laboratory[7]. UV-Visible spectroscopy and HPLC are employed to study the kinetic and reaction pathway of the photo catalytic degradation of the compounds. The size characterizations on nano TiO2 are measured using SEM-EDX and XRD. The nanoparticle catalyst sizes and poly dispersity index are studied. A combination method of sol-gel and hydrothermal is utilized in the preparation of nano crystalline TiO2[8]. The efficiency of the catalyst for the degradation of the four organo phosphorous and organo chloro compounds among the Ps and Is categories are graded[9]. The effects of variation in pH, solvent and ionic strength are carried out to optimize the oxidation reaction rates in aqueous medium.The method of employing nano crystalline TiO2 which is prepared in an eco-friendly method and using visible light for the decomposition of organic pollutants is potential in the view of green chemistry. The catalytic TiO2 powders can be produced in the laboratory with tunable microstructure and morphology by simply varying the parameters such as temperature, pressure, and process duration time, composition of precursors and pH of the solution. The major improvement over the degradation of the Ps, Hs and Is lies in using TiO2- visible light over the use of UV light. At the same time equal efficiency on the degradation rates are aimed at[10].The degradation kinetics involves the measurements of overall rate coefficient, the role of catalyst and as well as optimization of rate constant parameters under various medium effects such as pH and mass effect[11]. These data are essential to validate the removal and to evaluate the trend in the degradation rates of the four organic pesticide pollutants chosen in this work.
2. Experimental
2.1. Materials and Methods
- Titanium tetra isopropoxide was used as the precursor in the synthesis of nano TiO2 and was purchased from S.D.Fine chemicals Ltd, India with 99.9% purity. Triple distilled water was used in all solution preparations. The organic solvents used were purchased from Merck (Germany) without further purification. The pesticides are purchased from Fischer chemicals, India.
2.2. Preparation of Nano Crystalline TiO2
- Titanium dioxide Nanoparticles were synthesized by hydrolyzing titanium tetra isopropoxide in a mixture of 1:1 anhydrous ethanol and water. 9 ml of titanium tetra isopropoxide is mixed with 41ml of anhydrous ethanol (A). 1:1 ethanol and water mixture is prepared. (B) Solution A is added in drop wise to solution B and stirred vigorously for 2hrs. At room temperature hydrolysis and condensation are performed, using 1M sulphuric acid and stirred for 2 hrs. Then the ageing was undertaken for 12hrs. The gel was transferred into an autoclave and tightly closed, and the mixture was subjected to hydrothermal treatment at 353K for 24hrs. After filtration the solid residue was washed thoroughly with water and ethanol mixture, dried at 373K in an oven and calcined at 773K.
2.3. Size Characterizations of Nano Crystalline TiO2
- The white nano crystalline powders of TiO2 are characterized by Powder X-ray analysis was carried out using a Philips PW 1050/37 model diffractometer, operating at 40 kV and 30 mA. Cu Kα radiation with a wavelength of 1.54 Å and a step size of 0.02° in the 2θ range, 30-80 degrees was used. SEM-EDX experiments were carried out on a Hitachi S-3400 N instrument with EDX analyzer facility at 25℃. The samples are prepared by placing a drop of the colloidal metal solution on a carbon coated copper grid and allowing the solvent to evaporate. UV-Visible diffused spectra of the samples were recorded with Shimadsu UV 2450 model using BaSO4 white plate as reference.
2.4. Catalytic Study
- The catalytic degradation of the four organo pesticides was performed in all glass three necked round bottomed flask considered as the reactor. Tungsten lamp (100 w) was lit placing above the reactor. For every 10 mL of the aqueous solution of the pesticide, 1mg of catalyst was fed into the reactor and stirred continuously under visible light illumination and the progress of the reaction has been monitored with UV-Vis spectroscopy. Small aliquots of the sample was collected at regular intervals of time and filtered before the UV-vis spectra scan.
3. Results and Discussion
3.1. Kinetic Studies
- The aqueous solutions of pesticides with known concentration are irradiated with, visible light using tungsten lamp which is compatible with solar radiation both in the absence and presence of nano crystalline TiO2. After the start of the irradiation at regular intervals of time 5 mL aliquots of the solution are subjected to UV-spectral scan. The completion of the oxidative degradation of the pesticides is known from the gradual decrease of the absorbance value approaching the base line. The time of completion of oxidative degradation varies with the chemical nature of Ps, Hs and Is. From the time dependence UV-spectra profiles, OD Vs time plots are drawn. The limiting regions in the exponential plots indicate the completion of reactions. It was found that in the absence of nano-crystalline TiO2 the oxidative degradation was not detected on the substrates used in the present work. The photo catalytic disappearances of pesticides are found initiated only upon the incorporation of TiO2 nanoparticles. From the OD Vs time plots, the kinetic plots consisting of logOD0/ODt Vs time are generated. The first order rate coefficient (k) values are obtained from the slope values of the kinetic plots. The best fit linear plots are found in the kinetic analysis for the first order reaction under pseudo conditions only.
3.2. Spectral Studies
- The SEM-EDX patterns of synthesized TiO2 nanoparticles are given in Fig 1. It may be seen that, the crystalline phases of nanoparticles are also present in the mono disperse with an average size of 20 nm.Powder XRD analysis has been carried out to ascertain the nature of metallic particles. The diffractograms are carried out using dried powder. The XRD patterns showed that the metallic particles are anatase crystalline in nature. The observed reflection positions correspond to (101) as given in the Fig 2. The reflections of the anatase nanoparticles are different from the bulk components. The comparison of particle size between SEM-EDX and XRD data, the values obtained by XRD are slightly larger than the particle size deduced from SEM images.
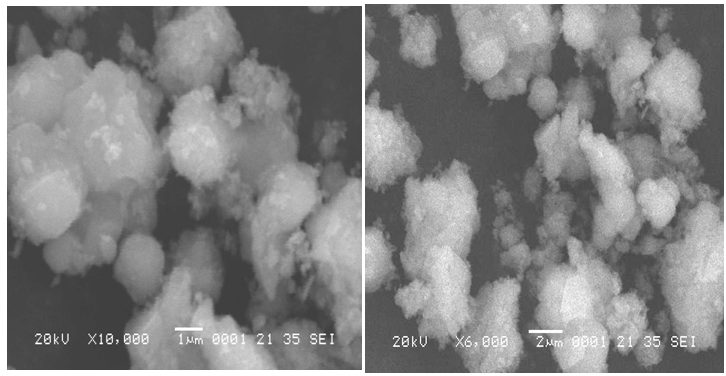 | Figure 1. Typical SEM photograph of nano crystalline TiO2 at 25℃ |
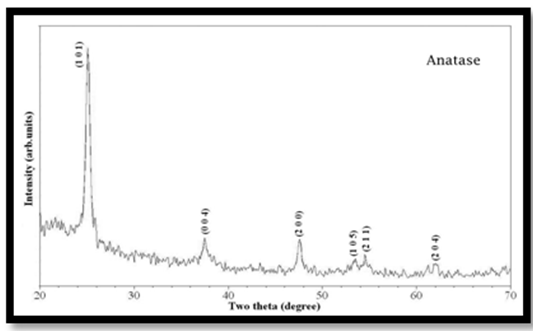 | Figure 2. Typical XRD patterns of nano TiO2 particles at 25℃ |
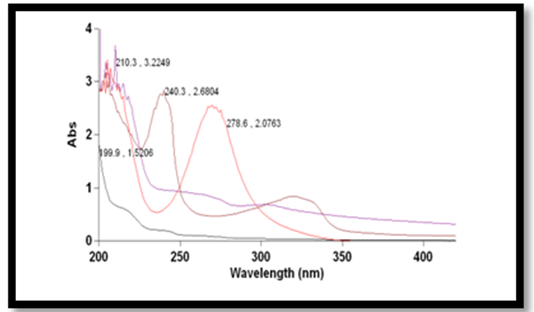 | Figure 3. Time dependent UV-visible spectra for the four pesticides Imidocloprid[Im], Endosulphon[Ch], Chloropyrifos[En] and Quinolphos[Qn] at 25℃ |
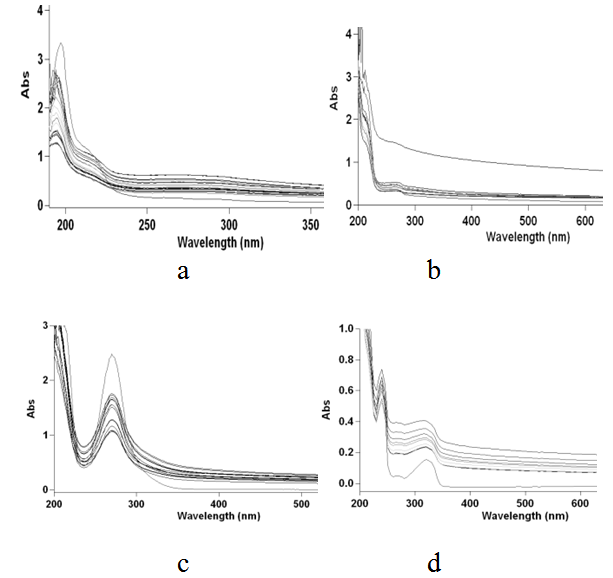 | Figure 4. Time Vs absorbance plot for four pesticides (a) Chloriopyrifos, (b) Endosulphon (c) Imidocloprid and (d) quinolphos at 25℃ |
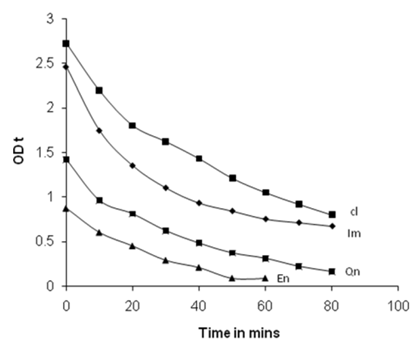 | Figure 5. Kinetic plots log[OD0/ODt] Vs time for four pesticides at 25℃ |
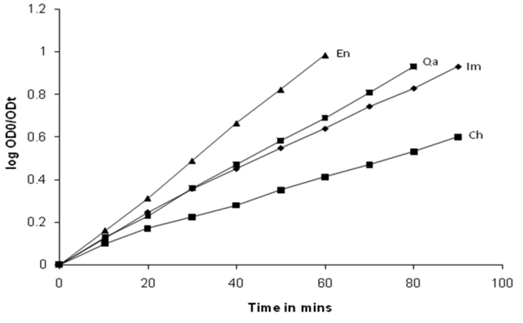 | Figure 6. Kinetic plots log[OD0/ODt] Vs time for four pesticides at 25℃ |
|
3.3. pH Effect
- The variation in the pH of the reaction medium definitely alters the overall rate coefficient of the oxidative degradation of the pesticides. This is because the ionization states of the surface of nano crystalline TiO2 stand pH controlled. Therefore pH variations strongly influence the adsorptive interaction of the substrate molecules onto the nano crystalline TiO2 surface. This is an important step in the photo catalyzed oxidations. It is known that hydroxyl radicals are formed at the catalytic surface when hydroxyl ion of TiO2 surface and the positive hole react. The positive holes serve as the oxidation sites at low pH (< 7.0) of the medium while hydroxyl radicals serve as the dominant species at neutral or high pH(>7.0) of the medium. In the alkaline medium, hydroxyl radicals are easily generated near the TiO2 surface. Hence there exists columbic repulsion between the negatively charged surface of the photo catalyst and the hydroxyl anions. This effect causes hydroxyl radical formation, resulting in the decrease in the hydroxyl radical formation. Hence, overall decrease in the photo oxidation rates is seen. In the fig 7 the pH profiles of the rate coefficients of the oxidative degradation are given. It may be seen that, in the alkaline pH there is a decrease in the rate coefficient values. The optimum pH values correspond to the maximum of the log k values. In the acidic pH, the optimum pH values are found to be 4.8, 5.2, 5.8 and 6.5 respectively for Ch, Qn, Im and En. These results reveal that the four pesticides are effectively and photo catalytically degraded at lower pH itself.
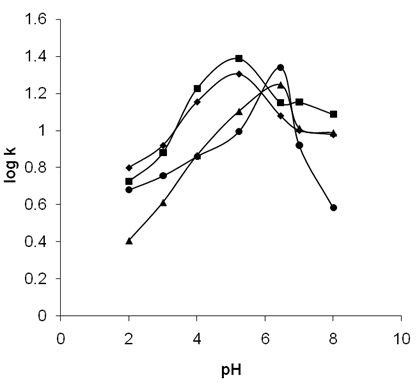 | Figure 7. The pH profile of the four pesticides at 25℃ |
3.4. Mass Effect
- The effects of nano crystalline TiO2 catalyst loading on the photo catalytic degradation of the pesticides in water have been investigated. In fig 8 the TiO2 mass dependent rate coefficient values are plotted. The slope values correspond to the optimum catalyst loading needed for the effective degradation of each of the four pesticides. In table 2 the threshold mass values are given. The increase in the amount of the catalyst generally increases the number of active sites on the catalyst surface. When the mass or concentration of the catalyst exceeds the optimum value then the degradation rate decreases. The catalyst particles agglomeration prevents the efficiency of the degradation and overall rate of oxidation is decreased.
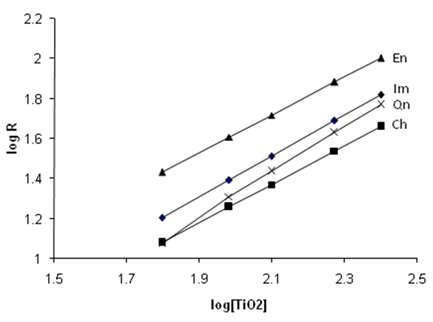 | Figure 8. The mass effect of the four pesticides at 25℃ |
4. Conclusions
- In conclusion, we have synthesized a nano crystalline TiO2 was synthesized by adopting combination of hydrothermal and sol gel method has been reported. The particle size results from HRSEM and XRD agree well with each other. The reaction conditions are optimized based on the various effects of pH, and catalyst loading on the photocatalytic oxidative degradation of the four pesticides. Instead of UV-light, visible light irradiation was employed which is a green chemistry reactions. Nano crystalline TiO2 as synthesized in the laboratory and size characterized to be 20±0.5 nm particles are chosen as the photo catalyst for the visible light interactions. The trend in the overall rate coefficient values among the four organo pesticides studied found to be Ch<Qn<Im<En. More detailed investigations of nanopar-ticle structure effects on the catalytic activity and their ap-plicability in other synthetic transformations are currently under investigation.
ACKNOWLEDGMENTS
- The authors are grateful to the authorities of University of Madras, Chennai and GKM college of Engineering and Technology, Chennai for providing all necessary facilities to carry out this work.
References
| [1] | Hubert Fallmann, Thomas Krutzler, Rupert Bauer, Sixto Malato, Julian Blanco, Catalysis Today, 1999, 54, 309-319 |
| [2] | Heinrich Wamhoff and Vera Schneder, J. Agric. Food. Chem, 2001, 47, 1730-1734 |
| [3] | Joseph Sherma, Journal of AOAC International, 2001, 84, 993-999 |
| [4] | S. Malato, and J. Caceres, Environ. Sci.Technol, 2001, 35, 4359-4366 |
| [5] | Ioannis K Kontantinou, Theophanis M. Sakellarides, Vasilis A. Sakkas, and Triantafyllos A. Albanis, Enivion, Science Technology, 2001, 35, 398-405 |
| [6] | Binbin Yu, Jingbin Zeng, Lifen Gong, Maosheng Zhang, Limei Zhang, Xi Chen, Talanta, 2007, 72, 1667-1674 |
| [7] | Amjad Islam, Muhammad Akhyar Farrukh, Attique ur Rahman, Fahim A. Aureshi and Sheraz Ahmed, American Eurasian, J. Agric & Envion Sci. 2010, 9(5), 514-518 |
| [8] | Periayasami Vijayan, Chinnathambi Mahendran, Chinnathambi Suresh, Kannan Shanthi, Catalysis Today, 2009, 141, 220-224 |
| [9] | Ioannis K Kontantinou, Theophanis M. Sakellarides, Vasilis A. Sakkas, and Triantafyllos A. Albanis, Enivion. Science Technology, 2001, 35, 398-405 |
| [10] | G.M. Madhu, M.A. Lourdu Antony Raj and K. Vasantha Kumar Pal, J. environ. Biol, 2009, 30(2), 259-264 |
| [11] | Antoine Piscopo, Didier Robert, Jean Victor Weber, Applied Catalysis B: Environmental, 2001, 35, 117-124 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML