-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2012; 2(1): 1-7
doi: 10.5923/j.nn.20120201.01
Synthesis and Physical Stability of Novel Au-Ag@SiO2 Alloy Nanoparticles
Orlando L. Sánchez-Muñoz 1, 2, Jesús Salgado 2, Juan Martínez-Pastor 1, Ernesto Jiménez-Villar 1, 2
1Instituto de Ciencia de Materiales, Universitat de València. C/ Catedrático José Beltrán Nº 2, 46980 Paterna Valencia, Spain
2Instituto de Ciencia Molecular, Universitat de València. C/ Catedrático José Beltrán Nº 2, 46980 Paterna Valencia, Spain
Correspondence to: Ernesto Jiménez-Villar , Instituto de Ciencia de Materiales, Universitat de València. C/ Catedrático José Beltrán Nº 2, 46980 Paterna Valencia, Spain.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The present study describes the synthesis of nanoparticles of silver-gold alloys and with their electrokinetic and spectroscopic characterisation. The synthesis was made in two steps. In the first step silver nanoparticles coated with silica (Ag@SiO2) were synthesised using a novel method assisted by laser ablation. The second step consisted on the introduction of KAuCl4 in the colloidal solution of Ag@ SiO2 nanoparticles in order to obtain silica-coated silver-gold alloy nanoparticles. The changes of colour and mean diameter of Ag@SiO2 nanoparticles caused by the introduction of the gold salt were found dependent on its concentration. Upon increasing [KAuCl4] the diameter of nanoparticles diminished and the monodispersity increased. The changes in the interparticle interaction potential as a function of [KAuCl4] and time were analysed using 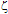 -potential values, calculated from their electrophoretic mobilities on the bases of Derjaguin-Laudau-Verwey-Overbeck (DLVO, UTDLVO) theory. The introduction of KAuCl4 produced a higher stability of the colloid and suggests an increase of the interaction energy barrier. However, as time after synthesis increases the barrier slightly decreases and stabilises at a plateau value. The absorbance measurements (Localised Surface Plasmon Resonance, LSPR) were studied as a function of [KAuCl4] and time. With increasing [KAuCl4] the main absorption band diminishes and red-shifts and a new broad band appears. For each value of [KAuCl4], upon increasing time, the two characteristic bands fuse into one and
-potential values, calculated from their electrophoretic mobilities on the bases of Derjaguin-Laudau-Verwey-Overbeck (DLVO, UTDLVO) theory. The introduction of KAuCl4 produced a higher stability of the colloid and suggests an increase of the interaction energy barrier. However, as time after synthesis increases the barrier slightly decreases and stabilises at a plateau value. The absorbance measurements (Localised Surface Plasmon Resonance, LSPR) were studied as a function of [KAuCl4] and time. With increasing [KAuCl4] the main absorption band diminishes and red-shifts and a new broad band appears. For each value of [KAuCl4], upon increasing time, the two characteristic bands fuse into one and 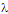 max diminishes in a linear fashion. Altogether, these data suggest that the co-reduced solutions of Ag⁻ and Au³⁻ salts at long time consists of alloy Ag-Au nanoparticles, and not a mixture of Ag and Au nanoparticles.
max diminishes in a linear fashion. Altogether, these data suggest that the co-reduced solutions of Ag⁻ and Au³⁻ salts at long time consists of alloy Ag-Au nanoparticles, and not a mixture of Ag and Au nanoparticles.
Keywords: Silver-gold nanoparticles, Core-shell nanoparticles, Nanoparticle synthesis, Electrokinetics of nanoparticles, Stability of nanoparticles, SiO2-coated nanoparticles
Cite this paper: Orlando L. Sánchez-Muñoz , Jesús Salgado , Juan Martínez-Pastor , Ernesto Jiménez-Villar , "Synthesis and Physical Stability of Novel Au-Ag@SiO2 Alloy Nanoparticles", Nanoscience and Nanotechnology, Vol. 2 No. 1, 2012, pp. 1-7. doi: 10.5923/j.nn.20120201.01.
1. Introduction
- The synthesis of nanostructured materials with useful and tunable properties is central to the development of nanoscale science and technology. Nanometre scale metal particles exhibit optical, electronic, chemical and magnetic properties of great technological and intellectual value.1 Among them, silver and gold nanoparticles (NP) show strong adsorption bands (Localised Surface Plasmon Resonance, LSPR) in the visible region which are absent for the individual atoms as well as in the bulk.2-3The frequency of the LSPR is strongly dependent on different properties of the NP.4-8 In turn, the optical and electrokinetic properties of metal NP are strongly influenced by their composition, size, shape, and surrounding environment, such as the proximity of other particles. The assembly of metallic NP presents interesting applications like single molecule detection using surface-enhanced Raman scattering (SERS)5,12-29 and nanoscale optical devices.30,31 On the other hand, the presence of a silica layer covering metal NP is important9,10 because it will separate the metal nano-spheres, avoiding touch with one another, and can also make metal particles more stable in air. More importantly, an outer silica coating provides an opportunity to tailor the electronic and/or optical properties of NP, to obtain 3D metallodielectric structures with a variety of enhanced functionalities.11The interesting colours observed in colloidal solutions of noble metals have led to extensive studies of their spectroscopic properties, in an effort to correlate their behaviour under different micro-environmental conditions,32-47 In this sense, Ag and Ag-Au alloy NP are particularly exciting and recent investigations report new modes of preparation48-50 and characterisation of their stability, morphology, size, composition and possible applications in nanoscience and nanotechnology.Silica-coated silver NP (Ag@SiO2) produced by a method assisted with laser ablation (ALA) are structured by a silver core and a porous silica shell,49-52 which in turn consists of the accumulation of small SiO2 nanoparticles (1-2 nm)49-50 In this paper we describe the synthesis and optical properties of alloy Au-Ag@SiO2 NP. The stability of the NP suspensions is also analysed within the framework of the Derjaguin-Laudau-Verwey-Overbeck (DLVO) theory.
2. Experimental Section
- Materials. The reagents used were AgNO3 (silver nitrate) 99%, from Paureac; KAuCl4 (potassium tetrachloroaurate (III)) 98%, from Sigma-Aldrich and Na2CO3 (sodium carbonate) 99%, from Fluka. All these chemicals were used as received without further purification or treatments and their solutions were prepared in de-ionized milliQ water.Synthesis Procedure. Step 1: Nearly monodispersed Ag@SiO2 nanoparticles were produced by the ALA method, using a third-harmonic (355 nm) Q-Switch Nd:YAG laser irradiation and a Silicon target in an aqueous solution of AgNO3 1.25x10-4 M48-50 ALA is a simple method for the fast (2 to 3 min), scalable synthesis of inert colloidal metal- silica nanoparticles in stable colloids. The method is based on the laser ablation of a solid target submerged in an aqueous solution of the metal salts, whose reduction gives rise to nanoparticles. In addition, ablation parameters, target materials and metal salts can be combined and controlled to influence the size, morphology and composition of nanoparticles48-50 Freshly synthesised Ag@SiO2 nanoparticles were filtered to eliminate bigger particles, using a NALGENE® filter with a Polietersulfona (PES) membrane and 0.2 µm pore size.Step 2: 8 mL of Ag@SiO2 nanoparticle suspension were distributed in 6 vials and each of them added a particular amount of KAuCl4, reaching final concentrations from 0.1x10-4 to 0.2x10-4 M. This procedure was carried out under continuous agitation of the sample and with slow additions of KAuCl4 (in steps of 0.02x10⁻⁴ M increments).Step 3: About five hours after addition of the gold salt, Na2CO3 was was added to reach a 0.5 mM concentration, in order to get pH stabilisation at
 8.5. The electrokinetic and spectroscopic characterisation for Ag@SiO2 and Au- Ag@SiO2 alloy nanoparticles were then performed by measuring the absorbance, size and
8.5. The electrokinetic and spectroscopic characterisation for Ag@SiO2 and Au- Ag@SiO2 alloy nanoparticles were then performed by measuring the absorbance, size and 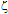 -potential as a function of time (after NP synthesis) and for each of the different KAuCl4 concentrations employed in step 2.Measurements of NP diameter. Small aliquots of each sample were transferred onto copper mesh grids, covered with a carbon film and let dry on air. A transmission electron microscope (JEOL, mod. JEM-1010, 100 kV accelerating voltage) with a digital MegaView III camera and “Analysis” software for image acquisition, was employed to take electron micrographs of the resultant Ag@SiO2 and Au- Ag@SiO2 alloy NP. From these electron micrographs the diameter of NP was measured using the ImageJ 1.40g software (National Institute of Health, USA).Spectroscopic Measurements. NP-sample solutions were transferred into 10 mm quartz cells and their absorption spectra were recorded using a UV-VIS Spectrophotometer (UV-250 1PC) from Shimadzu Corporation, Japan.Electrophoretic Mobility Measurements. The ZetaSizer NanoZS Zen3600 (Malvern Instruments Ltd., UK) was used to measure the electrophoretic mobilities (
-potential as a function of time (after NP synthesis) and for each of the different KAuCl4 concentrations employed in step 2.Measurements of NP diameter. Small aliquots of each sample were transferred onto copper mesh grids, covered with a carbon film and let dry on air. A transmission electron microscope (JEOL, mod. JEM-1010, 100 kV accelerating voltage) with a digital MegaView III camera and “Analysis” software for image acquisition, was employed to take electron micrographs of the resultant Ag@SiO2 and Au- Ag@SiO2 alloy NP. From these electron micrographs the diameter of NP was measured using the ImageJ 1.40g software (National Institute of Health, USA).Spectroscopic Measurements. NP-sample solutions were transferred into 10 mm quartz cells and their absorption spectra were recorded using a UV-VIS Spectrophotometer (UV-250 1PC) from Shimadzu Corporation, Japan.Electrophoretic Mobility Measurements. The ZetaSizer NanoZS Zen3600 (Malvern Instruments Ltd., UK) was used to measure the electrophoretic mobilities ( e) of each nanoparticle sample. NanoZS employs back scattering data detection (173° scattering angle) with a 4 mW He-Ne laser (633 nm). The electrophoretic mobilities were acquired using the M3-PALS technique, with a folded capillary cell (DTS1060). The
e) of each nanoparticle sample. NanoZS employs back scattering data detection (173° scattering angle) with a 4 mW He-Ne laser (633 nm). The electrophoretic mobilities were acquired using the M3-PALS technique, with a folded capillary cell (DTS1060). The 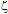 -potential values were calculated from the electrophoretic mobilities using the Henry´s approximation. Three measurements of each NP sample were made at 25 ± 0.1℃ to get average values.
-potential values were calculated from the electrophoretic mobilities using the Henry´s approximation. Three measurements of each NP sample were made at 25 ± 0.1℃ to get average values.3. Results and Discussion
- Pure Ag@SiO2 solutions are typically of a pale yellow colour (Figure 1 top, sample 1). This does not change in presence of Na2CO3 pH 8-8.5 (Figure 1 top, sample 1a). However, upon addition of KAuCl4 the colour changes gradually depending on the concentration of the gold salt and finally acquires a yellowish-red colour at the highest KAuCl4 concentration used (0.2 x 10⁻⁴ M, Figure 1 top, samples 2-7). We interpret these changes as being due to gold reduction and concomitant oxidation of silver from the NP core, leading to the formation of Au-Ag@SiO2 alloy NP (see below).
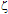 -potential measurements and their interpretation using the classical DLVO theory. Such a theoretical framework has been employed in colloid science to study particle-particle interactions, coagulation, sedimentation, filtration and the behaviour of electrolyte solutions53-56 This theory is based on the idea that pair-wise interactions arise from the interplay of attractive van der Waals forces (Fattr) and repulsive Coulomb forces (Frep) screened by Debye-Hückel ion clouds. Then, the dispersed colloid will be stable (non aggregated) for Frep >> Fattr and the total interaction potential between two nanoparticles (UTDLVO) can be expressed as the sum of electrostatic repulsion (Uelec) and the van der Waals attraction (Uvdw):53,54
-potential measurements and their interpretation using the classical DLVO theory. Such a theoretical framework has been employed in colloid science to study particle-particle interactions, coagulation, sedimentation, filtration and the behaviour of electrolyte solutions53-56 This theory is based on the idea that pair-wise interactions arise from the interplay of attractive van der Waals forces (Fattr) and repulsive Coulomb forces (Frep) screened by Debye-Hückel ion clouds. Then, the dispersed colloid will be stable (non aggregated) for Frep >> Fattr and the total interaction potential between two nanoparticles (UTDLVO) can be expressed as the sum of electrostatic repulsion (Uelec) and the van der Waals attraction (Uvdw):53,54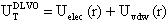 | (1) |
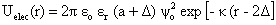 | (2) |
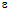 r is the permittivity of the medium,
r is the permittivity of the medium, 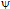 o is the potential at the particle surface, which can be estimated from the
o is the potential at the particle surface, which can be estimated from the 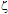 -potential measurements,57-61 is the inverse Debye length and
-potential measurements,57-61 is the inverse Debye length and 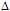 is the thickness of the Stern layer.Assuming that the particles are spherical and that the surface potential and the background ionic strength are constant, the van der Waals attraction potential (Uvdw) between the two particles can be calculated as54-56,58
is the thickness of the Stern layer.Assuming that the particles are spherical and that the surface potential and the background ionic strength are constant, the van der Waals attraction potential (Uvdw) between the two particles can be calculated as54-56,58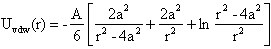 | (3) |
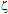 -potentials through measurements of electrophoretic mobility. For that we used Henry´s approximation, where f(
-potentials through measurements of electrophoretic mobility. For that we used Henry´s approximation, where f(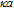 ) was calculated for
) was calculated for 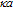
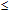 1.64Such an analysis was performed for the Ag@SiO2 NP immediately after their synthesis (sample 1), as well as after addition of KAuCl4 at the required concentrations (samples 2-4).The changes of the
1.64Such an analysis was performed for the Ag@SiO2 NP immediately after their synthesis (sample 1), as well as after addition of KAuCl4 at the required concentrations (samples 2-4).The changes of the 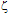 -potential of each sample are represented in Figure 2. The electrophoretic mobility increases (in absolute value) with [KAuCl4], from 2.34x10-8 m2V-1s-1 (sample 1) to 3.40x10-8 m2V-1s-1 (sample 2) and 3.64x10-8 m2V-1s-1 (sample 3). For sample 4, the increment of the electrophoretic mobility was smaller (3.35x10-8 m2V-1s-1).
-potential of each sample are represented in Figure 2. The electrophoretic mobility increases (in absolute value) with [KAuCl4], from 2.34x10-8 m2V-1s-1 (sample 1) to 3.40x10-8 m2V-1s-1 (sample 2) and 3.64x10-8 m2V-1s-1 (sample 3). For sample 4, the increment of the electrophoretic mobility was smaller (3.35x10-8 m2V-1s-1).
|
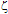 -potential are used to estimate the electrokinetic properties and colloidal stability of the NP suspensions. The interaction potentials between two particles can be calculated as a function of their separation from Eqs. 1-3.The changes in the interaction potential of the Au-Ag@SiO2 alloy NP as a function of time are shown in Figure 3. Before addition of KAuCl4 (samples 1 and 1a), the Ag@SiO2 NP are stable since the energy barrier is high enough to prevent aggregation. From the previous measurements we have inferred that the introduction of Au3+ in the solution is followed by incorporation of gold to the nanoparticles, likely accompanied by partial oxidation of silver:
-potential are used to estimate the electrokinetic properties and colloidal stability of the NP suspensions. The interaction potentials between two particles can be calculated as a function of their separation from Eqs. 1-3.The changes in the interaction potential of the Au-Ag@SiO2 alloy NP as a function of time are shown in Figure 3. Before addition of KAuCl4 (samples 1 and 1a), the Ag@SiO2 NP are stable since the energy barrier is high enough to prevent aggregation. From the previous measurements we have inferred that the introduction of Au3+ in the solution is followed by incorporation of gold to the nanoparticles, likely accompanied by partial oxidation of silver: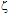 -potential. With time, the Au atoms that conform the surface layer could spread toward the interior of the NP, creating a radial distribution with a more homogeneous composition. This effect, in turn, would tend to diminish the
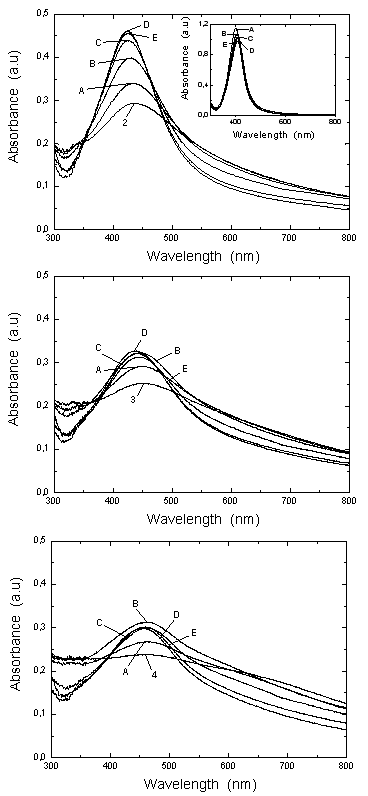
-potential. With time, the Au atoms that conform the surface layer could spread toward the interior of the NP, creating a radial distribution with a more homogeneous composition. This effect, in turn, would tend to diminish the 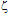 -potential, to be stabilized at a given value, as we observe.After 5 hour and addition of Na2CO3, the energy barrier increases significantly for all samples. Such an increase tends to be similar for all samples after longer times, reaching approximately the same value of UTDLVO.Optical properties of the Au-Ag@SiO2 alloy nanoparticles in Na2CO3 Solution. The absorbance of Ag@SiO2 and Au-Ag@SiO2 alloy NP as a function of time is given in Figure 4. Ag@SiO2 NP show a characteristic peak at ~403 nm (Figure 4, top, inset). The addition of Na2CO3 provokes a red shift of
-potential, to be stabilized at a given value, as we observe.After 5 hour and addition of Na2CO3, the energy barrier increases significantly for all samples. Such an increase tends to be similar for all samples after longer times, reaching approximately the same value of UTDLVO.Optical properties of the Au-Ag@SiO2 alloy nanoparticles in Na2CO3 Solution. The absorbance of Ag@SiO2 and Au-Ag@SiO2 alloy NP as a function of time is given in Figure 4. Ag@SiO2 NP show a characteristic peak at ~403 nm (Figure 4, top, inset). The addition of Na2CO3 provokes a red shift of 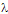 max which may be due to a higher accumulation of silica on the surface of NP which, as we have suggested above.71,72
max which may be due to a higher accumulation of silica on the surface of NP which, as we have suggested above.71,72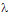 max of Au-Ag@SiO2 NP diminish as follows: sample 2, 434.0-424.5 nm; sample 3, 450.5-442.0 nm and sample 4, 465.0-458.5 nm. In parallel, the intensity of the principal absorption band increases, while the secondary broad band decreases up to a point where it cannot be observed. This time effects can be explained by the diffusion of Au from an initial external layer into the Ag core of the NP.
max of Au-Ag@SiO2 NP diminish as follows: sample 2, 434.0-424.5 nm; sample 3, 450.5-442.0 nm and sample 4, 465.0-458.5 nm. In parallel, the intensity of the principal absorption band increases, while the secondary broad band decreases up to a point where it cannot be observed. This time effects can be explained by the diffusion of Au from an initial external layer into the Ag core of the NP.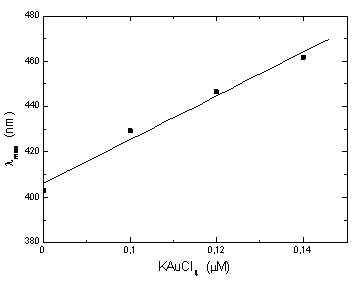 | Figure 5. Maximum absorbance wavelength as a function of [KauCl4], corresponding to data in the Figure 4. |
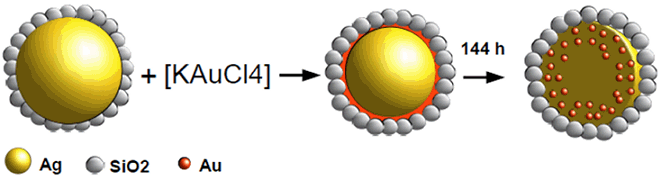 | Figure 6. Schematic representation of the synthesis and structure of Au-Ag@SiO2 alloy nanoparticles |
ACKNOWLEDGEMENTS
- This research was funded by grants as Invited Researcher from the University of Valencia, Spain (OLS) and Juan de la Cierva Program from the Ministry of Science and Innovation, Spain (EJ).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML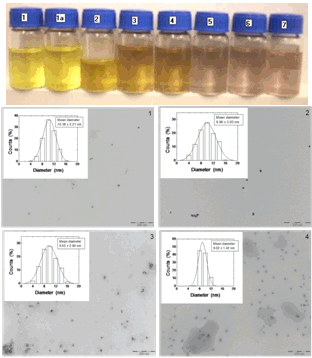
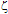 -potential of Ag@SiO2 (1, 1a) and Au-Ag@SiO2-alloy NP (2-4) as a function of time (A-5 hours; B-24 hours; C-48 hours; D-120 hours; E-144 hours). The plotted standard error was calculated from the errors of experimental measures of electrophoretic mobilities.
-potential of Ag@SiO2 (1, 1a) and Au-Ag@SiO2-alloy NP (2-4) as a function of time (A-5 hours; B-24 hours; C-48 hours; D-120 hours; E-144 hours). The plotted standard error was calculated from the errors of experimental measures of electrophoretic mobilities.
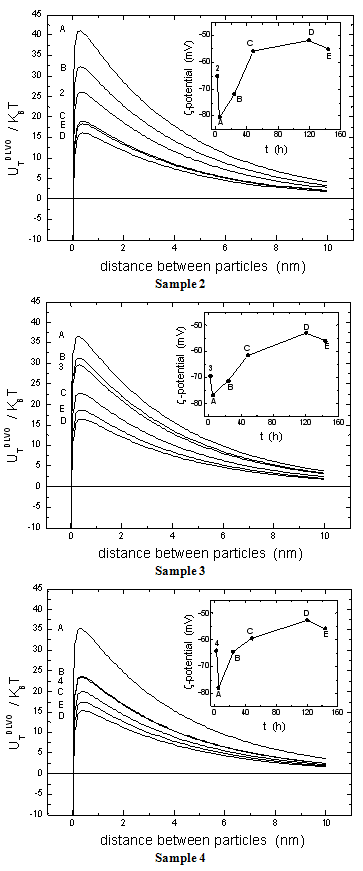
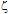 -potential changes as in Figure 2.
-potential changes as in Figure 2.