-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2011; 1(2): 40-42
doi: 10.5923/j.nn.20110102.07
Water Bactericidal Properties of Nanosilver- Polyurethane Composites
George Mulongo 1, Jolocam Mbabazi 2, P. Nnamuyomba 1, Song Hak-Chol 1
1Department of Chemistry, Gulu University, Gulu, P. O. Box 166, Uganda
2Department of Chemistry, Makerere University, Kampala, P. O. Box 7062, Uganda
Correspondence to: Jolocam Mbabazi , Department of Chemistry, Makerere University, Kampala, P. O. Box 7062, Uganda.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A technique for coating polyurethane with silver nanoparticles freshly prepared using a hydroxyethyl cellulose organic polymer is presented. The ensuing silver nanoparticle – polyurethane foam composite is found to possess such antibacterial properties as might be useful in water disinfection. With the help of electron microscopy and FTIR spectrophotometry, it was revealed that the adsorption of metallic silver onto the organic polymeric substrate could be attributed to silver nanoparticulate interaction with the nitrogen in the ─NH─ bond in polyurethane. Contrary to earlier reports that similar preparations had total bactericidal effects on water-borne microorganisms, a number of microbial analyses performed on Basillus Subtulis 15 and E. coli 36 as test bacteria in infected waters exhibited only 9 and 5 live percentages, respectively, after eluation through the composite-packed column.
Keywords: Nanosilver-Polyurethane Composite, Hydroxyethyl Cellulose, Bactericidal Properties
Cite this paper: George Mulongo , Jolocam Mbabazi , P. Nnamuyomba , Song Hak-Chol , "Water Bactericidal Properties of Nanosilver- Polyurethane Composites", Nanoscience and Nanotechnology, Vol. 1 No. 2, 2011, pp. 40-42. doi: 10.5923/j.nn.20110102.07.
Article Outline
1. Introduction
- This study is prompted by the necessity to continue to develop new techniques for contaminated water disinfection using methods other than conventional. It has been firmly established1,2 that the high reactivity associated with nanoparticles is due to their large surface-area to volume ratio. As a result, it has long been suspected that this unusual characteristic property might play a crucial role in such processes as water purification, especially in the increasingly common situations where acute scarcity renders fresh water an extremely essential but scarce resource3,4. It is in this light that silver nanoparticles, silver ion (Ag+) and silver nanocomposites have been the subject of extensive investigations to establish whether they might exhibit antibacterial and antiviral properties in real biological systems5,6. Even in extremely low concentrations, silver nanoparticles have been shown to demonstrate enhanced antibacterial activity via binding to microbial DNA, which in turn greatly deactivates bacterial multiplication7. Consequently, it is thought that situations which result in further increase in surface area by, for example, the introduction of single nanoparticles2 onto porous material such as polyurethane, might lead to further enhancement of their biological activity. Such modifications have been referred to as nanocomposites8. In this respect, polyurethane foams have been particularly singled out for this purpose owing to their possession of the carbamate (_N(H)COO_) group which would be expected to facilitate occlusion with nanoparticulate surfaces9,10. Raman, FE- SEM/EDS and ICP-AAS data confirms that silver nanoparticles are stable on polyurethane foam base and remain occluded even after several aqueous washings. The organic polymeric material hydroxyethyl cellulose2 has been selected as a suitable substrate for the enhancement of the nanoparticulate binding to the carbamate group in polyurethane.The present study specifically reports the synthesis and characterisation of silver nanoparticle polyurethane coated composites prepared with the help of hydroxyethyl cellulose, an organic polymer with unique high electrical charge density and high viscosity properties2, as well as the results of our tests regarding their antibacterial effectiveness in the treatment of municipal wastewaters.
2. Materials and Methods
- Reagents and Instruments: The reagents used in this study were all of analytical grade quality. The silver(I) nitrate employed was supplied by Aldrich and was used as received. Exclusive use of distilled de-ionised water was observed throughout the entire investigation, including the preparative and quantification processes. Hydroxyethyl cellulose (HEC), an organic polymer earlier incorporated as a suitable medium for the synthesis of silver nanoparticles2, was used at a QP value of 10000H, a viscosity range of (6000-7000) x 10-3 N s m-2 and at a carefully controlled temperature of 25oС. Preparation of silver nanoparticles was conducted as earlier described2. Various chunks of polyurethane foam were obtained from locally available sources. Scanning electron micrographs (SEMs) were taken on a JEOL 30-kV instrument. Infrared spectra in neat and/or KBr pellet forms were obtained on a Shimadzu 8400 S FTIR spectrophotometer with a range of 4500-350 wave- numbers.Preparation of Nanosilver-Polyurethane Foam (Ag- PU) Composite: Sheets of polyurethane foam were soaked in silver nanoparticle solution, previously prepared as in Ref. 2, overnight. The sheets were washed repeatedly with distilled-de-ionised water to remove any loosely adsorbed ions and subsequently allowed to dry under ordinary air currents. The resulting Ag-PU composite foams were made to undergo both electron microscopy and FTIR spectrophotometry as aids to further investigation. Biological Activity Tests: The presence of the microorganisms E. coli 36 and Bacillus subtilis 15 bacteria are often considered to be good indicators of faecal contamination in water. Nutrient broth was used as the growing medium. The cultures were centrifuged, the cells washed and suspended in distilled-deionised water, and allowed to attain respective final concentrations of 2 x 105 and 1.6 x 105 CFU/mL.Test-tube Test: 10mL aliquots of E. coli 36 and Bacillus subtilis 15 suspensions in distilled-deionised water were drawn into previously sterilised test-tubes. 1 cm3 cubes of the stiff and dry nanosilver-polyurethane foam composite were cut with the help of sterile scissors and immersed, one piece in each of the test suspensions. After 10 min, the foams were drawn out from the tubes, each transferred into another empty tube and pressed to squeeze out the supposedly treated eluate water. The pour plate method was carried out with this eluate on 1-, 100- and 10,000-fold dilutions. Plating using 0.05mL of solution was also performed for the initial CFU count and on the eluate obtained after pressing uncoated polyurethane. The plates were in each case allowed to incubate for 12 h at 37℃.
3. Results and Discussion
- These studies indicate that polyurethane foam, left soaking overnight in silver nanoparticle solution, changes colour from white to yellow. Scanning electron micrographic images of polyurethane foam prior to and after silver nanoparticle-coating are shown in Figure 1. The polyurethane maintained its morphology upon coating with silver nanoparticles. The particles are uniformly adsorbed onto the polyurethane surface. Similar SEM images taken of samples in the absence of HEC do not exhibit any nanoparticle attachment9. On close examination of the SEM surfaces of the samples used in this study, however, one can easily see adsorbed nanosilver particles, in spite of several times of washing with distilled-deionised water (Figure 1b). We may therefore affirm that the adhesive HEC character (Figure 1a), here likened to a kind of glue, is responsible for the physico-chemical surface adhesion of silver particles onto the PU foam. FTIR spectroscopic studies reveal that binding is due to the interaction between the nitrogen of the polyurethane ─NH─ bond and the nanosilver particles. The spectral evidence of the coated and uncoated PU samples in the relevant electromagnetic region is displayed in Figure 2. Consequent upon nanoparticle coating, there is a significant shift in the characteristic absorption peak at υmax= 3301 cm-1 corresponding to urethane linkage (-NH stretching bond), in the apparent constancy of all the other peaks.
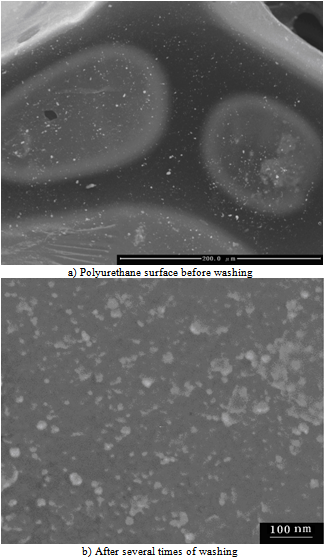 | Figure 1. Effect of Nanosilver colloid solution treatment on the Scanning Electron Micrographic pictures of Polyurethane foam |
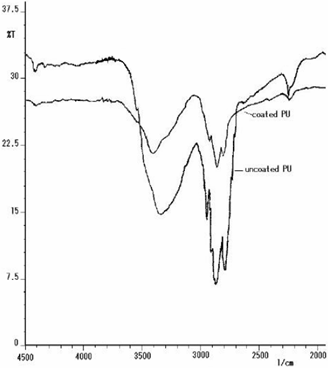 | Figure 2. FTIR spectra of coated and uncoated polyurethane |
|
4. Conclusions
- This study shows that synthetic nanosilver particles adsorbed onto polyurethane foam exhibit a marked activity against certain species of water-borne bacteria. Continuation of this work is already in progress in an effort to establish to what extent such nanoparticle composites could be considered in matters of water purification.
ACKNOWLEDGEMENTS
- The authors would like to thank Gulu University for financial support, and to the Faculty of Science at the same locality for playing host to the project.
References
| [1] | Ichinose N., Ozaki Y. and Kaghu S., 1992, Superfine Particle Technology, Springer, New York |
| [2] | Mulongo George, Mbabazi Jolocam and Song Hak-Chol, 2011, Synthesis and Characterisation of Silver Nanoparticles using High Electrical Charge Density and High Viscosity Organic Polymer, Res. J. Chem. Sci., 1(4), 18 |
| [3] | Stoimenov P. K., Klinger R. L., Marchin G. L. and Klabunde K. J., 2002, Metal Oxide Nanoparticles as Bactericidal Agents, Langmuir, 18, 6679 |
| [4] | Zhang L. Z., Yu J. C., Yip H. Y., Li Q., Kwong K. W., Xu A. W. and Wong P. K., 2003, Ambient Light Reduction Strategy to Synthesise Silver Nanoparticles and Silver-Coated TiO2 with Enhanced Photocatalytic and Bactericidal Activities, Langmuir, 19, 10372 |
| [5] | Shameli K, Ahmad M. B. and Yunus W. M. Z. W., 2010, Green Synthesis of Silver/Montmorillonite/Chitosan Bionanocomposites using the UV Irradiation Method and Evaluation of Antibacterial Activity. Int. J. Nanomedicine, 5, 875 |
| [6] | Ahmad M. B., Shameli K. and Yunus W. M. Z. W., 2009, Synthesis and Antibacterial Activity of Silver/Montmorillonite Nanocomposites, Res. J. Biol. Sci. 4(9), 1032 |
| [7] | Holt K. B. and Bard A. J., 2005, Interaction of Silver (I) ions with the Respiratory Chain of E. Coli: An Electrochemical and Scanning Electron Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag+, Biochemistry, 44, 13214 |
| [8] | Evanoff D. D., Jr. and Chumanov G., 2005, Synthesis and Optical Properties of Silver Nanoparticles and Arrays, Chem Phys. Chem. 6(7), 1221 |
| [9] | Prashant Jain and Pradeep T., 2005, Potential of Silver Nanoparticle-Coated Polyurethane Foam As an Antibacterial Water Filter, Biotechnology and Bioengineering, 90, 59 |
| [10] | Yeo S. Y., Lee H. J. and Jeong S. H., 2003, Preparation of Nanocomposite Fibers for Permanent Antibacterial Effect, J. Mat. Sci., 38, 2143 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML