-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2011; 1(2): 36-39
doi:10.5923/j.nn.20110102.06
PbS - Nanoparticles Embedded in Polymer Matrix: Preparation and Characterization
P. K. Singh, S. K. Tomar and B. Bhattacharya
Material Research Laboratory, Department of Physics, School of Engineering & Technology, Sharda University, G. Noida, 201306, India
Correspondence to: P. K. Singh, Material Research Laboratory, Department of Physics, School of Engineering & Technology, Sharda University, G. Noida, 201306, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Formation of nano-sized PbS in PEO – polymer matrix at room temperature is being reported. SEM showed the particle size distribution while TEM affirmed the formation of nano particulates of PbS. The size distribution was further supported by UV/VIS absorption of the colloid. At room temperature a wide variation in the size of the particles was observed depend on sulfuration and salt concentration. The detailed characterization of these nano - PbS doped in polymer electrolyte are also discussed.
Keywords: PbS nanopartciles, Polymer electrolytes, SEM, TEM
Cite this paper: P. K. Singh, S. K. Tomar and B. Bhattacharya, PbS - Nanoparticles Embedded in Polymer Matrix: Preparation and Characterization, Nanoscience and Nanotechnology, Vol. 1 No. 2, 2011, pp. 36-39. doi: 10.5923/j.nn.20110102.06.
Article Outline
1. Introduction
- Small inorganic semiconductor crystallites in the form of colloids have attracted researchers due to their very interesting properties[1]. The experimental verification of the transformation between molecular and bulk properties in small aggregates is being highly persuaded. Large number of studies has been reported so far on the formation of nano crystallites of semiconductors in zeolite and polymer matrix as well[2-5]. The various properties of nano-crystals has become the topic of both theoretical[6,7] and experimental interest[8-12]. In most of the studies, nano-crystalline semiconductors belonging to the II-VI group have been taken up while the studies related to IV-VI are rather scanty. PbS is an important member of this family. It is one of the earliest materials studied for its interesting photoconductive properties which has been exploited by many researchers[13]. The basic problem associated with colloidal semiconductors like CdS, PbS is that the lifetime of the photogenerated electron/hole pair is very short. Therefore, for fast electron transfer reactions, a very high concentration of reagents is required. However, such high concentrations destabilize the colloid. In order to prevent such a stability problem, dispersion of colloids into polymer matrix is a novel alternative. Keeping this thing in mind we have doped the nano-PbS paricles into an ion conducting polymeric matrix. The polymer electrolyte chosen for the present study is PEO:NH4I (80:20) which is predominantly a proton conducting polymer electrolyte[14,15] and maintain good ionic conductivity and mechanical stability.
2. Experimental
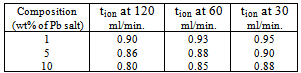
- To prepare polymeric membranes dispersed with semiconductor, Pure PEO (Mol. wt. 6x105, Aldrich) was dissolved in dehydrated methanol to which stoichiometric ratio of anhydrous NH4I (purity, Aldrich) was added to give PEO:NH4I weight ratio as 80:20. This solution was thoroughly stirred at 400C for 5 hours for complete complexation. After that we added a dilute solution of lead acetate to it drop by drop. As soon as the drop falls, H2S was bubbled through the viscous solution (at 30 ml./min.) for 2-3 minutes which resulted in the formation of PbS. A fresh Pb-acetate drop was added and sulfuration was done as above.This continued till the desired weight ratio of Pb-acetate gets added.The bubbling rate (sulfuration) was controlled by flowmeter. Colloidal particles of PbS was formed in PEO-matrix by following reactionPb(CH3COO)2+H2S → PbS+2 CH3COOHThe desired weight percent of Pb-acetate salt (1, 5, 10 wt%) and bubbling rate of H2S ( 30, 60, 120 ml/min.) were varied to obtain the different films. This PbS-dispersed polymer electrolyte viscous solution was poured into petri dishes for solution casting into film form. After drying it in room ambient for several days, the film were finally dried under vacuum to eliminate all traces of solvent. These nano-PbS embedded in polymer matrix were characterized with JEOL JSM-5800 LV scanning electron microscope, PHILLIPS TEM instrument (Model EM-CM 12). The band gap of the dispersed PbS was evaluated by UV/visible absorption spectra using Hitachi (model U-3400) spectrophotometer. For calculation of the energy band gap the equation, as given below, was employed αhυ =A(hυ-Eg)nwhere hυ denotes the energy of the incoming photon in electron volt, α is the absorption coefficient of the material, Eg is the energy band gap, A is a constant and the exponent n is 1/2 for direct band gap materials[16].
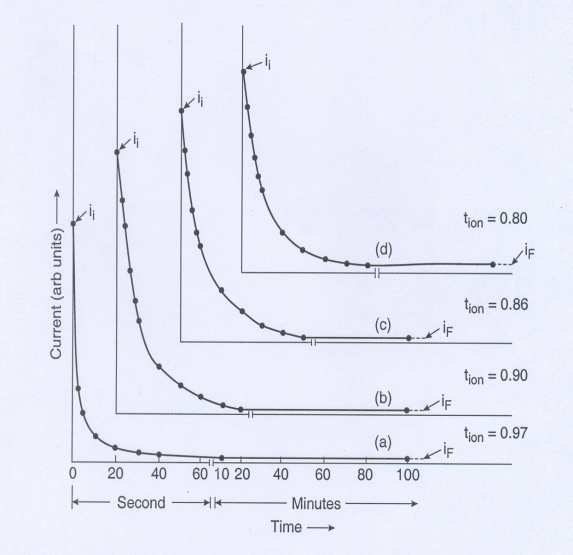
3. Results and Discussions
- To confirm the presence/formation of lead sulfide we have recorded the XRD diffraction patterns of PbS doped polymer films. The known prominent peaks of bulk PbS are at 2θ=26.1 and 30.3. Unfortunately, near these angles some PEO-peaks are also present and it is difficult to distinguish. Hence, we carried out XRD measurement at a slow scan rate. A typical XRD pattern in the range 2θ=24-320 is shown in figure 1 for films containing 5 and 10 wt% of PbS. The results of an approximate deconvolution of these peaks into peaks assigned either to PEO or PbS are also shown in figure1. The dominant particle sizes determined from this data using Scherrer formula[17] are lies between 10-15 nm. The SEM photographs of different (PEO:NH4I)+PbS films with different composition and sulfuration are given in figure 2. It was obvious from the comparison of figures 2 a and 2 b that more was the amount of Pb-acetate in the starting solution, bigger aggregated PbS gets dispersed.
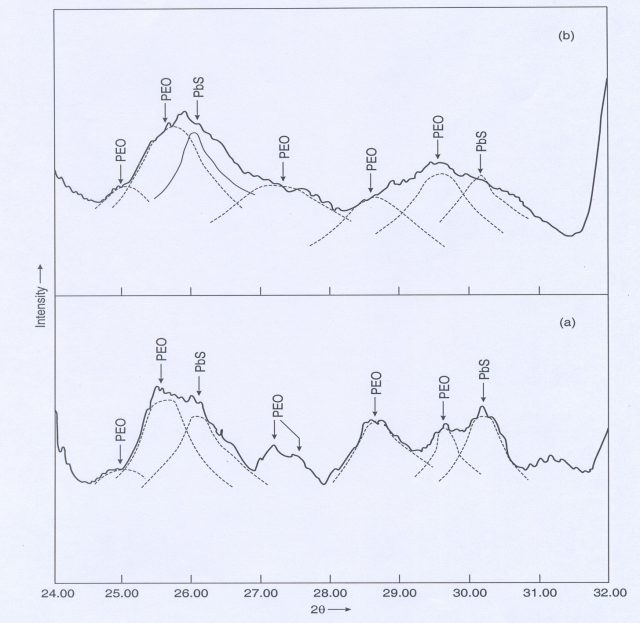 | Figure 1. The deconvoluted XRD peaks of (PEO:NH4I)+PbS composite membrane containing (a) 5 wt% and (b) 10 wt% of PbS |
 | Figure 2. SEM micrographs of (a) (PEO:NH4I)+1 wt% PbS (b) (PEO:NH4I)+5 wt% PbS at 30 ml/min. and (c) (PEO:NH4I)+1 wt% PbS at 120 ml/min. polymer electrolyte films |
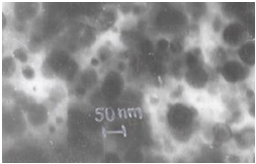 | Figure 3. TEM micrograph showing size distribution of PbS nanoparticles in polymeric matrix |
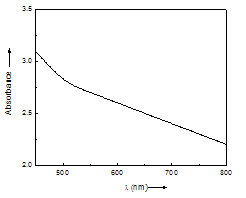 | Figure 4. The absorption spectra of PEO:NH4I+1 wt% PbS polymer electrolyte film prepared at 120 ml/min |
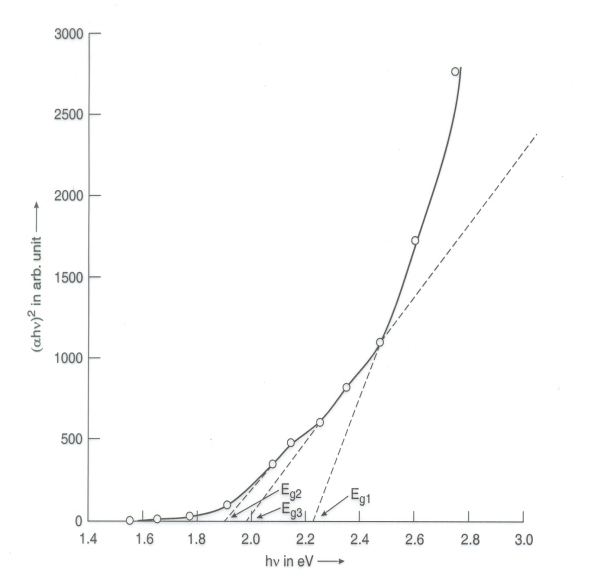 | Figure 5. The (αhυ)2 Vs hυ plot of PEO:NH4I+1 wt% PbS polymer electrolyte film prepared at 120 ml/min |
|
4. Conclusions
- Hence, we have successfully prepared the PbS nano particles in the PEO - polymer electrolyte matrix using chemical sulfuration method. A size distribution of particles was observed in XRD/SEM which were supported by UV/VIS optical absorption spectra and TEM micrographs. Low salt concentration and bubbling rate was found to be suitable for the formation of smaller (nano) particulates of PbS. Further we have shown that the doping of semiconductor could be a novel approach to modify the electrical properties of polymer electrolytes.
ACKNOWLEDGEMENTS
- This work is supported by DST project (SR/S2/CMP-0065/2010) government of India.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML