-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Nanoscience and Nanotechnology
p-ISSN: 2163-257X e-ISSN: 2163-2588
2011; 1(1): 22-29
doi:10.5923/j.nn.20110101.04
Physical Stability of Novel Au-Ag@SiO2 Alloy Nanoparticles
Orlando L. Sánchez-Muñoz1, 2, Jesús Salgado2, Juan Martínez-Pastor1, Ernesto Jiménez-Villar1, 2
1Instituto de Ciencia de Materiales, Universidad de Valencia. Pol. La Coma, 46071, Paterna Valencia, Spain
2Instituto de Ciencia Molecular, Universidad de Valencia. Pol. La Coma, 46071, Paterna Valencia, Spain
Correspondence to: Ernesto Jiménez-Villar, Instituto de Ciencia de Materiales, Universidad de Valencia. Pol. La Coma, 46071, Paterna Valencia, Spain.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The present study deals with nanoparticles synthesis of silver-gold alloys and their electrokinetic-spectroscopic characterization. The synthesis consisted in two steps. The first step: synthesis of silver nanoparticles coated of silica using the novel assisted laser ablation method. The second step: Introduction of a [KAuCl4] in the colloidal solution of silver nanoparticles, previously synthesized, in order to obtain the nanoparticles of silver-gold alloy coated with silica. The colour change and their mean diameter size, caused by the introduction of the gold salt, were found dependent on the [KAuCl4] added in solution. The diameter size diminishes at to increasing [KAuCl4] and the monodispersity is more accented for the samples with high [KAuCl4]. The changes in the interparticle interaction potential, as a function of the [KAuCl4] and time, were analysed using the ζ-potential calculated from their electrophoretic mobilities based on the Derjaguin-Laudau-Verwey-Overbeck (DLVO, 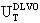 ) theory. The introduction of a [KAuCl4] provokes the energy barrier increases, suggesting a higher stability, but as the time elapses, it undergoes a slight decrease and spreads to a plateau for all the samples in the same way. The absorbance measurements (Localized Surface Plasmon Resonance (LSPR)) were studied increasing [KAuCl4] and as a function of the time. With increasing [KAuCl4] the absorption bands diminishes and appear a broadband red-shifted. Upon the time, the two bands becomes fused into one and the λmax starts to diminish in a linear fashion for each [KAuCl4]. Thus, our data suggest that the co-reduced solution at long time consists of alloy nanoparticles and not a mixture of Ag@SiO2 and Au nanoparticles.
) theory. The introduction of a [KAuCl4] provokes the energy barrier increases, suggesting a higher stability, but as the time elapses, it undergoes a slight decrease and spreads to a plateau for all the samples in the same way. The absorbance measurements (Localized Surface Plasmon Resonance (LSPR)) were studied increasing [KAuCl4] and as a function of the time. With increasing [KAuCl4] the absorption bands diminishes and appear a broadband red-shifted. Upon the time, the two bands becomes fused into one and the λmax starts to diminish in a linear fashion for each [KAuCl4]. Thus, our data suggest that the co-reduced solution at long time consists of alloy nanoparticles and not a mixture of Ag@SiO2 and Au nanoparticles.
Keywords: Silver-gold Nanoparticles, Coreshell Nanoparticles, Synthesis Nanoparticles, Electrokinetic Nanoparticles, Stability of Nanoparticles, SiO2-Capped Nanoparticles
Cite this paper: Orlando L. Sánchez-Muñoz, Jesús Salgado, Juan Martínez-Pastor, Ernesto Jiménez-Villar, Physical Stability of Novel Au-Ag@SiO2 Alloy Nanoparticles, Nanoscience and Nanotechnology, Vol. 1 No. 1, 2011, pp. 22-29. doi: 10.5923/j.nn.20110101.04.
Article Outline
1. Introduction
- The synthesis of nanostructured materials with useful and tuneable properties is central to development in nanoscale science and technology. Nanometer scale metal particles exhibit optical, electronic, chemical and magnetic properties of great aesthetic, technological and intellectual value.1 Characteristically, silver and gold nanoparticles, exhibit a strong adsorption band in the visible region and this is indeed a small particle effect, since they are absent in the individual atoms as well as in the bulk.[2,3] The frequency of the LSPR is strongly dependent of different properties of the nanoparticles.4-8 The optical and electrokinetic properties of metal nanoparticles are strongly influenced by their composition, size, shape, and surrounding environment, such as, the proximity of other particles. The silica layer on the metal nanoparticles is important,[9,10] because it will separate metal spheres, avoiding touch with one another and can also make metal particles are more stable in air. More importantly, the existence of an outer silica coating could provides us with an opportunity to tailor its electronic, or optical properties to obtain 3D metallodielectric structures with a variety of enhanced functionalities.[11] The assembly of metallic nanoparticles presents interesting optical properties such as, single molecule detection using surface-enhanced Raman scattering (SERS)[5,12-29] and nanoscale optical devices.[30,31] Colloidal solutions of noble metal show characteristic colours that have received considerable attention from researchers. The interesting colours observed in colloidal solutions have led to extensive study of their optical spectroscopic properties in an effort to correlate their behaviour under different microenvironmental conditions. [32-47] The reason for the present excitement in Ag and Ag-Au alloy nanoparticles research is due to the new mode of preparation,[48-50] their stability, morphology, size, composition and its role in nanoscience and future nanotechnology. This Ag@SiO2 nanoparticles produced by assisted laser ablation (ALA) method are structured by a silver core and a porous silica shell,[49-52] which in turn, consists of the accumulation of small SiO2 nanoparticles (1-2 nm).[49,50] The aim of this paper is highlight the discussion in relation to the synthesis and stabilization phenomenon of the Au-Ag@SiO2 alloy nanoparticles. The stabilization phenomenon has been accounted in the light of the DLVO theory and experimental aspects of the optical properties will be discussed.
2. Experimental Section
- Materials. The reagents were purchased from, AgNO3 (silver nitrate 99%, Paureac), KAuCl4 (potassium tetrachloroaurate (III) 98%, Sigma-Aldrich) and Na2CO3 (sodium carbonate 99%, Fluka). All these chemicals were used as received without further purification or treatments and their solutions prepared in de-ionized milliQ water.Synthesis Procedure. Nearly monodispersed in diameter size Ag@SiO2 nanoparticles were produced by ALA method, using a third-harmonic (355 nm) Q-Switch Nd:YAG laser irradiation of a Silicon target in a aqueous solution of silver.[48-50] The ALA is a simple method for the fast (2 to 3 min), scalable synthesis of inert colloidal metal-silica nanoparticles in stable colloids. The method is based on the laser ablation of a solid target submerged in an aqueous solution of the metal salts, whose reduction will give rise to nanoparticles. In addition, ablation parameters, target materials, and metal salts can be combined and controlled to influence the size, morphology and composition of nanoparticles.[48-50] Experimental Design. Step 1: To synthesis Ag@SiO2 nanoparticles was used a concentration 1.25x10-4 M of AgNO3. After synthetised Ag@SiO2 nanoparticles, these were filtered to eliminate bigger particles, using a NALGENE® filter with Polietersulfona (PES) membrane with 0.2 µm pore size. Step 2: later on, 8 mL of Ag@SiO2 nanoparticles suspension were distributed in each recipients (6 in totals) and added to them several [KAuCl4] from 0.1 to 0.2x10-4 M, with step of 0.02. This process is carried out maintaining a continuous agitation of the sample and a slow adding of [KAuCl4]. Step 3: finally, a [Na2CO3] of 0.5 mM were added to the Au-Ag@SiO2 alloy nanoparticles to get a pH stabilization at
 8-8.5. The electrokinetic and spectroscopic characterization for Ag@SiO2 and Au-Ag@SiO2 alloy nanoparticles was performed measuring the absorbance, size and ζ-potential as a function of the time and [KAuCl4].Diameter Size Measurements. Small samples were transferred onto a copper mesh grid covered with a carbon film and let to dry. A transmission electron microscope (JEOL, mod. JEM-1010, 100 kV accelerating voltage) with a digital MegaView III camera and a ¨Analysis¨ software for the image acquisition, was employed to take the electron micrographs of the resultant Ag@SiO2 and Au-Ag@SiO2 alloy nanoparticles. From these electron micrographs and using the ImageJ 1.40g software (National Institute of Health, USA) the diameter size were measured.Spectroscopic Measurements. Nanoparticle sample solutions were transferred into a quartz cell 10 mm width and their absorption spectrum were recorded using a UV-VIS Recording Spectrophotometer (UV-250 1PC) from Shimadzu Corporation, Japan. Electrophoretic Mobility Measurements. A commercial device known as ZetaSizer NanoZS Zen3600 (Malvern Instruments Ltd., UK) was used to measure the electrophoretic mobilities (μe) of each nanoparticle sample solution. The electrophoretic mobility measurements were made using the M3-PALS technique, with a folded capillary cell (DTS1060). NanoZS is based on the back scattering data detection (173° scattering angle) and uses 4 mW He-Ne laser (633 nm). The ζ-potential values were calculated from the electrophoretic mobility measurements using the Henry´s approximation. Three measurements of each nanoparticle sample solution were made at 25 ± 0.1 ℃ to get average values.
8-8.5. The electrokinetic and spectroscopic characterization for Ag@SiO2 and Au-Ag@SiO2 alloy nanoparticles was performed measuring the absorbance, size and ζ-potential as a function of the time and [KAuCl4].Diameter Size Measurements. Small samples were transferred onto a copper mesh grid covered with a carbon film and let to dry. A transmission electron microscope (JEOL, mod. JEM-1010, 100 kV accelerating voltage) with a digital MegaView III camera and a ¨Analysis¨ software for the image acquisition, was employed to take the electron micrographs of the resultant Ag@SiO2 and Au-Ag@SiO2 alloy nanoparticles. From these electron micrographs and using the ImageJ 1.40g software (National Institute of Health, USA) the diameter size were measured.Spectroscopic Measurements. Nanoparticle sample solutions were transferred into a quartz cell 10 mm width and their absorption spectrum were recorded using a UV-VIS Recording Spectrophotometer (UV-250 1PC) from Shimadzu Corporation, Japan. Electrophoretic Mobility Measurements. A commercial device known as ZetaSizer NanoZS Zen3600 (Malvern Instruments Ltd., UK) was used to measure the electrophoretic mobilities (μe) of each nanoparticle sample solution. The electrophoretic mobility measurements were made using the M3-PALS technique, with a folded capillary cell (DTS1060). NanoZS is based on the back scattering data detection (173° scattering angle) and uses 4 mW He-Ne laser (633 nm). The ζ-potential values were calculated from the electrophoretic mobility measurements using the Henry´s approximation. Three measurements of each nanoparticle sample solution were made at 25 ± 0.1 ℃ to get average values.3. Results and Discussion
- The colour change caused, by reduction of gold salts, was found dependent on their concentration in solution. The pure Ag@SiO2 solution turned to light yellow; in contrast, increasing the [KAuCl4] the Au-Ag@SiO2 solution turned yellowish red.
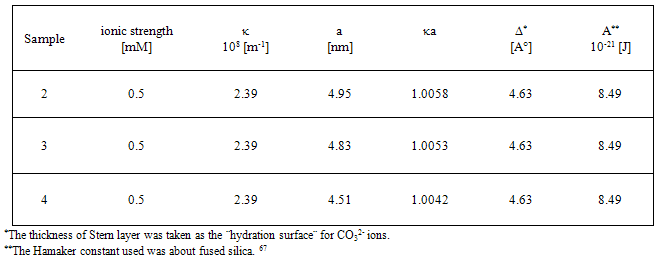
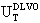 ) can be expressed as the sum of electrostatic repulsion (Uelec) and the van der Waals attraction (Uvdw),[53,54]
) can be expressed as the sum of electrostatic repulsion (Uelec) and the van der Waals attraction (Uvdw),[53,54] 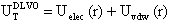 | (1) |
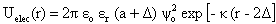 | (2) |
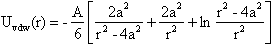 | (3) |
|
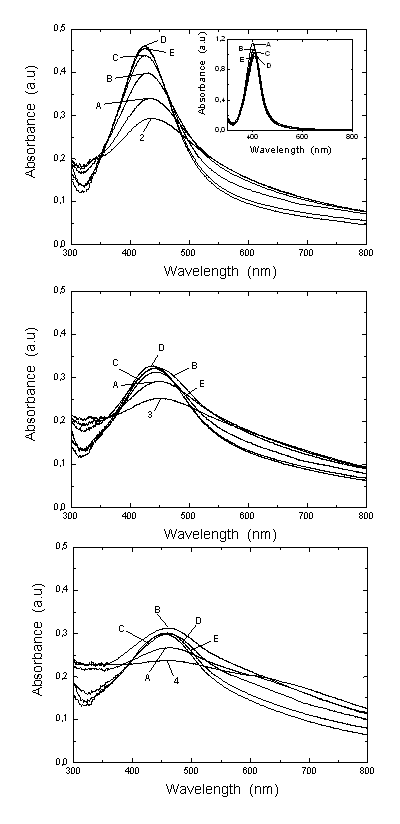
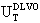 .Optical Response of the Au-Ag@SiO2 Alloys Nanoparticles in Na2CO3 Solution. The absorbance measurements of Ag@SiO2 and Au-Ag@SiO2 alloy nanoparticles systems are provided in Figure 4. The absorbance spectra (inset) display that the maximum wavelength (λmax) for Ag@SiO2 system is red shifted (sample 1A-E) showing a characteristic peak at 403 nm. As was commented, the Na2CO3 addition could cause a higher accumulation of silica on the surface of nanoparticles which provoke a red shift of λmax.[71,72]
.Optical Response of the Au-Ag@SiO2 Alloys Nanoparticles in Na2CO3 Solution. The absorbance measurements of Ag@SiO2 and Au-Ag@SiO2 alloy nanoparticles systems are provided in Figure 4. The absorbance spectra (inset) display that the maximum wavelength (λmax) for Ag@SiO2 system is red shifted (sample 1A-E) showing a characteristic peak at 403 nm. As was commented, the Na2CO3 addition could cause a higher accumulation of silica on the surface of nanoparticles which provoke a red shift of λmax.[71,72]  | Figure 5. Wavelength corresponding to the maximum absorbance for varying the [KAuCl4] in the Figure 4 |
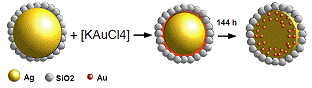 | Figure 6. Schematic representation for the synthesis process of Au-Ag@SiO2 alloy nanoparticles |
4. Conclusions
- The synthesis of nanostructured materials with useful and tuneable properties is central to development in nanoscale science and technology. The understanding of the electronic absorption and dynamics in individual nanoparticles is essential before assembling them into devices, which is essentially the future goal of the use of nanostructured systems. For that reason, electrokinetic and spectroscopic characterization should be a first step before the application of these nanoscale Ag@SiO2 and Au-Ag@SiO2 alloy particles. In this work has been to understand more clearly the composition, structure and electronic properties of Ag@SiO2 and Au-Ag@SiO2 alloy nanoparticles. On the other hand, is established that the addition of KAuCl4 and consequently formation of Au-Ag@SiO2 results in greater stability of the colloidal solution. Additionally, it is demonstrated that the introduction of Na2CO3 causes a further increase in colloidal stability.
ACKNOWLEDGEMENTS
- This research work was funded by grants as Invited Researcher from the University of Valencia, Spain. It is also a part of the activities for the Laboratory for Material and Optoelectronic Devices appointed by the Institute of Materials Science and for the Biomembranes Group appointed by the Institute of Molecular Science. To the Ministry of Science and Innovation (Juan de la Cierva Program), Spain.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML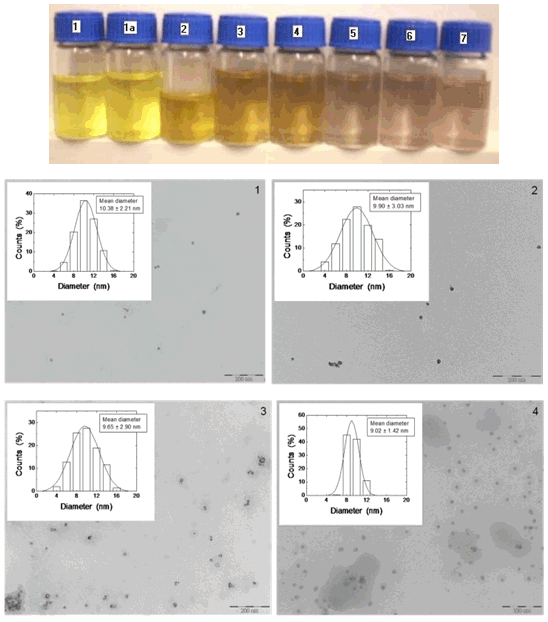

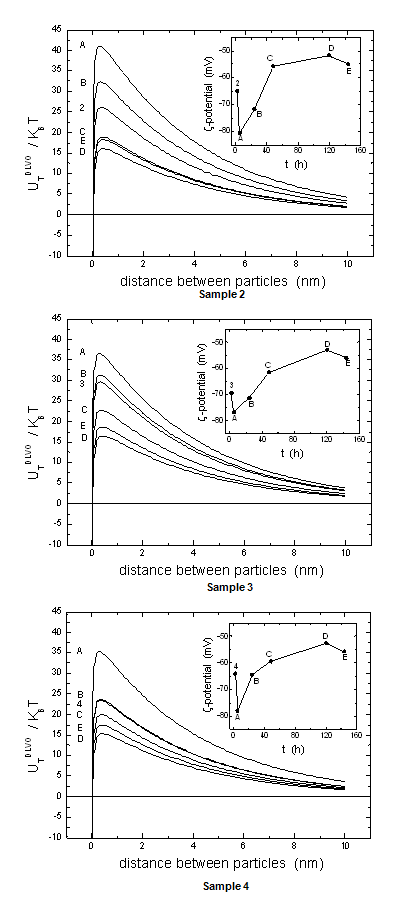
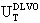 ) for Au-Ag@SiO2 alloy nanoparticles as a function of the time (A-5 hours [Na2CO3]; B-24 hours; C-48 hours; D-120 hours; E-144 hours). The inset shows the ζ-potential changes as in Figure 2
) for Au-Ag@SiO2 alloy nanoparticles as a function of the time (A-5 hours [Na2CO3]; B-24 hours; C-48 hours; D-120 hours; E-144 hours). The inset shows the ζ-potential changes as in Figure 2