-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2019; 8(1): 1-8
doi:10.5923/j.neuroscience.20190801.01

Psychotropic Effects Related to Withdrawal from "Odontol" in White Mouse Mus musculus Swiss
Rigobert-Espoir Ayissi Mbomo1, Mefo Foka Gloria1, Samuel Boris Tene Tadoum2, Elisabeth Sylvie Ngoa Manga3, Elisabeth Ngo Bum4, Alfred Kongnyu Njamnshi2
1Department of Biological Sciences, High Teacher Training College, University of Yaoundé I, Cameroon
2Department of Internal Medicine and Specialties, Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon
3Department of Animal Biology and Physiology, Faculty of Sciences University of Yaoundé I, Cameroon
4Department of Biological Sciences, Faculty of Sciences University of Ngaoundéré, Cameroon
Correspondence to: Rigobert-Espoir Ayissi Mbomo, Department of Biological Sciences, High Teacher Training College, University of Yaoundé I, Cameroon.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Depression and anxiety are among the most common of the central nervous system disorders in psychiatry. They are the predominant symptoms during withdrawal from several psychoactive substances and are also considered as important relapse factor. The aim of the present study was to evaluate the psychotropic effects of withdrawal from a well-known artisanal spirit in Cameroon (Odontol) in mice. Thus during 15 days, male white mice Mus musculus Swiss weighting between 18 to 29 g were exposed to Odontol). Subsequently, they were suddenly withdrawn from this beverage and depression-like behaviors in the paradigms of forced swimming (FST) and tail suspension tests (TST) and anxiety-like behaviors in the paradigm of the light/dark test (L/D) were evaluated. The tests were performed at different days after a withdrawal over a 24 days period. The more marked results at day 8 after the withdrawal from Odontol showed a significant (p< 0.001) decrease of the time of immobility’s occurrence in the FST (61%); a significant (p< 0.01) decrease of immobility’s occurrence (37%) as well as total immobility time in the TST (38%) compared to mice of the control group treated by tap water. In the L/D test, results showed that withdrawal from Odontol induced a significant (p< 0.001) decrease of the first latency of mice in the test group to leave the light compartment for the dark compartment. A significant decrease (p< 0.001) of the time spent in the light compartment of the L/D box was observed during withdrawal (57%). Odontol withdrawal significantly (p< 0.001) increased about 71% the time spent in the dark compartment of the L/D test when compared to control group mice. At day 24, these Odontol withdrawal’s induced effects seems to be reversed with the values of 73.2 ± 5.5 s and 101 ± 5.03 s respectively for the immobility delay and the total immobility time in FST; 34.2 ± 9 s and 123.8 ± 8 s respectively for the immobility delay and the total immobility time in the TST; 10 ± 4.2 s, 63.6 ± 11.4 s, and 171.6 ± 23 s for the latency, the time spent in the illuminated compartment and in the dark compartment respectively. As previously stated by Steru and Porsolt, by decreasing the occurrence of immobility while increasing the total immobility time in the FST and TST, or by decreasing the time spent in the light compartment of the L/D box as presented by Crawley withdrawal after long term exposure to Odontol induces a high depression and anxiety-like behaviors in mice for several days, and these effects seems to decrease 24 days after the withdrawal.
Keywords: Depression, Anxiety, Withdrawal, "Odontol", Psychoactive substance
Cite this paper: Rigobert-Espoir Ayissi Mbomo, Mefo Foka Gloria, Samuel Boris Tene Tadoum, Elisabeth Sylvie Ngoa Manga, Elisabeth Ngo Bum, Alfred Kongnyu Njamnshi, Psychotropic Effects Related to Withdrawal from "Odontol" in White Mouse Mus musculus Swiss, Research in Neuroscience , Vol. 8 No. 1, 2019, pp. 1-8. doi: 10.5923/j.neuroscience.20190801.01.
Article Outline
1. Introduction
- Now days mental and psychiatric disorders become more and more common in our societies. The international community recognizes that these "invisible" disabilities are one of the most neglected but most critical issues for achieving international development goals. Associated with drug abuse disorders, they significantly contribute to distress, suffering, disruption of function, and risk of death [1]. They affect individuals, families, communities, schools, and the entire health care system. Each year, approximately 14% of the population uses health services for mental illness, with anxiety and mood disorders such as depression being the most common problems [2]. Depression is a mental disorder characterized by sad mood, loss of interest or pleasure, feelings of guilt or worthlessness, disturbed sleep or appetite, tiredness or lack of concentration [3]. Depression becomes a public health concern because it is associated with various pathologies [4]. Independently of this important co-morbidity, it significantly affects the quality of life and has a major impact on the physical and relational capacities of the individual, so it becomes very difficult to assume his or her social and professional roles [5]. According to a WHO publication (2004), depression will become the second leading cause of disability worldwide in 2020 after cardiovascular disease [6]. Anxiety which is a mental state of trouble and agitation, with an indefinable feeling of insecurity, a fear without object [7], represents the most common category of psychiatric disorders followed by depressive disorders. It affects nearly 15% of the general population during their lifetime [8]. The global prevalence of anxiety disorders is 7.3%, ranging from 5.3% in African cultures to 10.4% in Europe [9]. Anxiety significantly affects the quality of life of people (difficulty working, doing daily chores and having good relationships with others). Moreover, in many cases, these disorders generate financial difficulties and great personal instability [7]. Psychoactive substances users frequently suffer from mental health disorders [10]. Abusive or addictive use of licit drugs such as alcohol and tobacco, or illicit drugs such as heroin or psycho-stimulants is often accompanied by psychiatric disorders. The co-occurrence in the same individual of drug abuse disorder and another psychiatric disorder leads to a worsening of the prognosis, a greater difficulty in the management and more serious consequences on the socio-occupational, professional and relational levels [11]. Alcohol is one of the most common addictive substances in the world. Alcohol consumption is now a social issue in many countries [12]. According to the WHO in 2011, alcoholism is one of the leading causes of death in the world, and it is estimated at about 2.5 million deaths per year, more particularly 320000 young people between 15 and 29 years die from alcohol related, representing up to 9% of the total mortality in this age group. Globally, 6.2% of men's deaths are alcohol-related, compared to 1.1% of women's deaths [13]. In Africa, the consumption of alcoholic beverages continued with the installation of breweries and distilleries in the 1960 s [14]. However, alcohol consumption has actually increased with local manufacturing of spirits by artisanal methods. In Cameroon, artisanal production of alcoholic beverages is widespread, and the consumption of these drinks represents 40 to 60% of total alcohol consumption [15]. There are several varieties of such beverages depending of different regions of the country ('' Oofofo '', '' Hâ '', '' Odontol '', '' Malamba '', '' bil bil '' and many others). The "Odontol" beverage, made for decades in Cameroon, mainly in the South, East and Center regions is very popular because of its very attractive price [16]. The production of this traditional drink is an important income source for peasants [15], however it creates significant havoc. In 2016, among many other cases, 27 people in districts located in the upper Nyong division died and about 40 others were hospitalized after "Odontol" consumption [16]. With this tragedy, the production of "Odontol" was banned by the administrative authorities. However, for many, the brutal ban of this alcohol is not enough; it would first of all be necessary to make people aware of the dangers related to this drink [17]. It is within the framework of this sensitization that the Animal Physiology Laboratory of the Higher Teacher Training College of the University of Yaoundé I has taken a closer interest in this spirit. For this purpose, parallel works on memory and addiction are conducted. The present study will focus on the psychotropic effects associated with "Odontol" withdrawal. The main objective is to evaluate the consequences of withdrawal after prolonged exposure of mice to "Odontol", on the expression of anxiety and depressive disorders.
2. Material and Methods
2.1. Animals
- These experiments were conducted on male white mice Mus musculus Swiss strain weighing between 20 and 25 g. Animal provided from a colony bredded at the higher teacher training college, subject to a natural photoperiod cycle, ambient temperature of 25 ± 2°C and 45–55% relative humidity. Mice were housed in cages made of plastic basins covered with a wire mesh serving as a receptacle through which beverages (water or "Odontol") and food were delivered ad libitum. All animal handling and sacrifice procedures were carried out in strict compliance with the guidelines of the National Guide to Ethics applied in Cameroon (FWA-IRB00001954).
2.2. Pharmacological Agents
- "Ondontol" and tape water were respectively given to each group of mice. "Odontol" used was produced from a traditional method of distillation and rectification based on palm wine, sugar and bark of Garcinia lucida.
2.3. Pharmacological Treatments
- Mice were separated into two main groups of 10 mice each. The test group received a solution of "Odontol" as the only beverage during 15 consecutive days at the end of which they were brutally withdrawn. Another group receiving only tap water as a beverage served as a control group.
2.4. Behavioural assessments
- At the end of 15 days treatment, mice were transported to the laboratory 24 hours before the beginning of the behavioural tests, to allow them to adapt to the experimental environment [18]. They were then tested for depression and anxiety at different intervals: baseline (day 0), day 3, day 8, and day 24.
2.4.1. Assessment of the Depressive State
2.4.1.1. Forced Swim Test
- PrincipleThe forced swimming test or Porsolt test is a pharmacological test widely used to assess the potential antidepressant properties of new molecules. It is makes possible by forcing the mice to individually swim in a cylindrical glass tank, without the possibility of escape. After few minutes, the proportion of swimming time decreases in favor of the immobility behaviour, representing the proportion of time when the animal floats passively by making only the movements necessary to maintain its head out of the water. This immobility time is an indicator of the depressive state or animal’s [19].ProcedureThe procedure used is similar to those initially described by Porsolt and his colleague in 1977[19] and taken up by several other authors [20,21]. A pretest was performed during which each mouse was introduced for 6 minutes into a glass cylinder (40 cm high x 20 cm in diameter) filled with tap water above a height of 20 cm of at room temperature. A test of 6 minutes was carried out under the same conditions 24 h later. After each swimming session, the water was changed and the animal was quickly dried with a towel and placed under a heat source and then returned to the home cage. The behaviour of each animal was video recorded for 360 s to measure the following parameters:- Delay of appearance of the first immobility in seconds;- Total duration of the immobility over the last four minutes in seconds.
2.4.1.2. Tail Suspension Test
- PrincipleLike the Porsolt swimming test, the tail suspension test is a predictive test for depression. Originally developed by Steru and co-workers in 1985, and intensively used by behavioural studies laboratories, it is also measures immobility time in rodents. It is based on the fact that the rodent suspended by the tail at a support raised at a certain height of the ground, develops a deflection or early agitation which gives way after a moment to an almost permanent immobility and adopts a behaviour of despair [22,23].ProcedureThe device used was rectangle-shaped (60 x 40 x 20 cm) raised to 70 cm above the ground. Each mouse was individually suspended by the tail to the upper part of the device and the behaviour video recorded for a cut off time of 360 s. The measured parameters were the time of onset of the first immobility (in seconds) and the total duration of immobility (in seconds) during the last four minutes.
2.4.2. Assessment of Anxiety Level
2.4.2.1. Light/Dark Test
- PrincipleThis test was initially developed by Crawley and Goodwin in 1980 to evaluate aversive reactions of mice vis-à-vis a strongly lit compartment opposite to a dark compartment in the same box. Widely used by many researchers now, it is based on the natural tendency of rodents to avoid lighted areas, and spontaneous exploratory behaviour of new environments. Here, mice tend to spend more time in the dark compartment and explore less the lighted compartment. The light/dark test is used as a model of acute anxiety such as "unconditioned fear". It also makes it possible to quickly and easily assess animal behaviours related to anxiety as well as their modifications by pharmacological agents [24,25].ProcedureThe light/dark box (59 x 50 x 14 cm) was made of wood and consisted of two chambers of different texture, sizes and colors. The small room (16 x 50 cm) entirely covered with walls black painted. The largest room (34 x 50 cm) was opened on the top, with walls white painted and lit by daylight. An entrance (6 x 6 cm) located in the wall between the two rooms allowed the animals to transit from one room to another. For the test, mice were individually placed in the center of the lit room facing the entrance and allowed to explore the whole apparatus for 5 minutes [26]. The following parameters were noted and recorded in the cut off time of 300 seconds:- The latency to enter the dark compartment in seconds;- The time spent in the lit compartment in seconds;- The time spent in the dark compartment in seconds.
2.5. Data Analysis
- The results were expressed as mean ± SEM (Standard Mean Deviation), and then the data processed using the STATISCA software 6.0. The variables were analyzed by ANOVA one way, followed by a post hoc analysis with FISHER LSD test if there was a difference between the analyzed groups. Probabilities less than 5% (p < 0.05) were considered statistically significant.
3. Results
3.1. Assessment of the Depressive State
3.1.1. Forced Swim Test
3.1.1.1. Effect of "Odontol" Withdrawal on the Time of Immobility Onset
- The result shows no difference in latency of the first immobility in the control group and test group at day 0 (P) and day 3 (D). However, it was observed 61% significant decrease (p< 0.001) in the occurrence of first immobility in mice withdrawn from 58.4 ± 3.5 s in the control group to 22.8 ± 4.2 s on day 8. On day 24 (Q), the decrease in the latency observed on day 8 was reversed, thus this parameter significantly increased (p< 0.001) up to 73.2 ± 5.5 s.Each bar represents the mean time of the first immobility occurrence (s) ± S.E.M. n = 10. *** P < 0.001, significant difference compared to the control group treated with tap water; +++ p< 0.001, significant difference compared to the test group at day T. Tm: control group; P: test group at day 0; D: test group at day 3; T: test group at day 8; Q: test group at day 24.
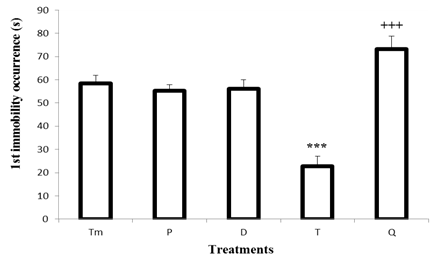 | Figure 1. Effect of "Odontol" withdrawal on the occurrence of the first immobility in the forced swimming test |
3.1.1.2. Effect of "Odontol" Withdrawal on the Total Immobility Time
- Figure 2 illustrates the effects of withdrawal on the total immobility time in the Porsolt swimming test. No significant difference was observed between the total immobility time in the control group mice and mice withdrawn to "Odontol" at day 0 (P), day 3 (D). However on day 8, result indicates a significant (P<0.01) decrease of 39% from 129 ± 12.6 s to 77.8 ± 3.4 s of the total immobility time when compared to control group. On day 24 (Q), even observed light increase of immobility time, the difference was not statistically significantly when compared to the effect observed at day 8.
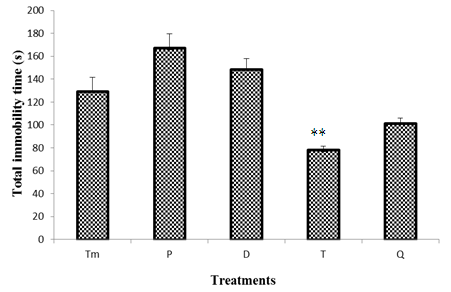 | Figure 2. Effect of "Odontol" withdrawal on total immobility time in the forced swimming test |
3.1.2. Tail Suspension Test
3.1.2.1. Effect of "Odontol" Withdrawal on the Time of Immobility Onset
- Figure 3 shows the effects of "Odontol" withdrawal on the immobility occurrence in the tail suspension test. The results show a significant difference (p <0.001) between the immobility appearance in the control group mice (79 ± 13 s) and the test group at day 0 (P) (38.2 ± 3 s), day 3 (D) (51.8 ± 4 s) and day 24 (Q) (34.2 ± 9 s) representing a decrease of 52%, 34%, 57%, respectively.
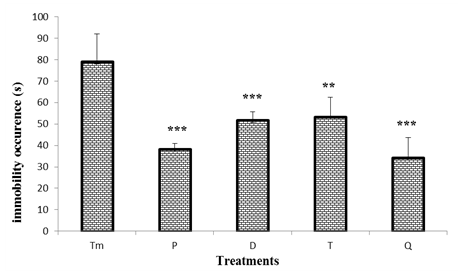 | Figure 3. Effect of "Odontol" weaning on the occurrence of the first immobility in the tail suspension test |
3.1.2.2. Effect of "Odontol" Withdrawal on the Total Immobility Time
- Figure 4 represents the effect of "Odontol" withdrawal on the total duration of immobility in the tail suspension test. In this figure there is no-significant difference between the total duration of the immobility of the control mice and that of the test mice at days P (day 0), D (day 3) and T (day 8). However, a significant increase (p <0.01) of 38% in the duration of the immobility was observed at day Q (day 24), since this parameter passed from 76.4 ± 14 s in the animals of the control group to 123.8 ± 8 s in animals of test group at day Q (day 24).
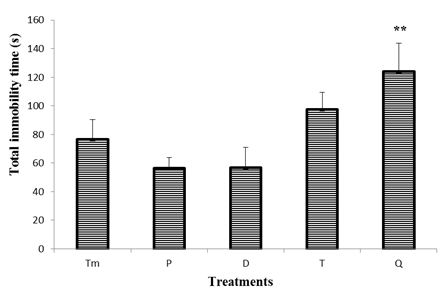 | Figure 4. Effect of "Odontol" withdrawal on total immobility time in the forced swimming test |
3.2. Assessment of Anxiety Level
3.2.1. Light/Dark Test
3.2.1.1. Effect of "Odontol" Withdrawal on the Latency in the Light / Dark Test
- As it can be seen in figure 5, the first latency to leave the lit compartment and to the dark compartment in the control group is significantly different (p <0.001) when compared to test group treated by "Odontol" at the beginning of the withdrawal (P). Thus it was observed a progressive decrease of latency from 18.2 ± 5.8 s in the control group to 7 ± 0.7 s at day 8 (T). At day 24, animals of the test group exhibited a slight significant increase (p <0.05) up to 10 ± 4.2 s when compared to test animal at day 8.
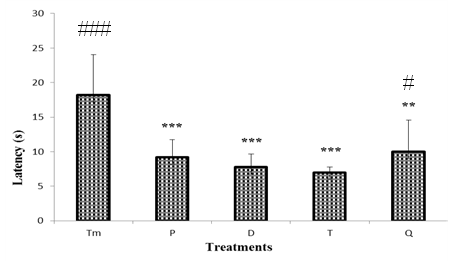 | Figure 5. Effect of "Odontol" withdrawal on latency in the Light/Dark test |
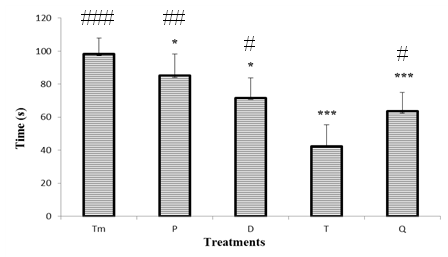
3.2.1.2. Effect of "Odontol" Withdrawal on Time Spent in the Dark Compartment
- Animals of control group spent 173.8 ± 11 s in the dark compartment. At the beginning of the withdrawal, it was observed a significant (p < 0.01) increase of the time spent in the dark compartment as this parameter increases from 211 ± 13 s at day 0 to 244.2 ± 13 s at day 8. However, compared to day T (day 8), there was a significant decrease (p< 0.001) to 171.6 ± 23 s of the time spent in the dark compartment at day Q (day 24).
 | Figure 6. Effect of "Odontol" withdrawal on the time spent in the dark compartment in the Light/Dark box |
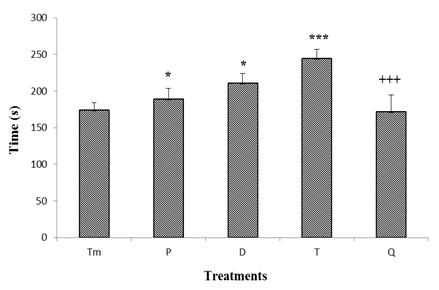
3.2.1.3. Effect of "Odontol" Withdrawal on Time Spent in the Lit Compartment
- As presented in figure 7, there was a significant decrease of the time spent in the lit compartment at the beginning of weaning. The value of the time spent in the illuminated compartment thus decreased from 98.4 ± 9.5 s in the control group to 85.2 ± 13 s, 71.6 ± 12.1 s and 42.4 ± 13 s in the test group at day 0 (P), day 3 (D) and day 8 (T) of withdrawal respectively. At day 24 (Q) of withdrawal, compared to the 8th day, the time spent in the lit compartment significantly increased at 63.6 ± 11 s.
 | Figure 7. Effect of "Odontol" withdrawal on the time spent in the lit compartment in the Light/Dark box |
4. Discussion
- The psychotropic effects of "Odontol" withdrawal were studied using mice models of depression (forced swimming and tail suspension tests) and anxiety (light/dark test). The forced swimming test or Porsolt model is a recognized and reliable pharmacological test, predictive of antidepressant activity [19]. Our results indicate a significant decrease in the immobility occurrence on day 8 after the withdrawal in comparison with mice of the control group. These results suggest that withdrawal induces the appearance of depressive-like behaviours in test mice as demonstrated by [20,21], since a decrease in the immobility time in the forced swimming test is a sign of a depression increase. The non-significant difference in the total duration of immobility could be explained by the fact that several factors may influence the immobility time in the forced swimming such as age, weight or time of day [27]. Previous studies using this test to assess the depressive state of animal during withdrawal to a known alcohol (ethanol), reported that withdrawn mice had an increased immobility time in the forced swimming test, thus revealing depression induction in animals [28]. As a result, the observed condition appears to be caused by withdrawal and needs time to manifest [29]. Some studies have shown that increased immobility in withdrawn mice in the forced swimming test is associated with depressive state and reduced hippocampal neurogenesis [30,31]. Others suggest that the depressive-type behaviours observed during alcohol withdrawal is associated with a reduction in brain-derived neurotrophic factor (BDNF), which is responsible for neuronal development [32]. The tail suspension test as well as the forced swimming test measures the duration of immobility of the animal also considered here as an index of resignation [20, 21]. The main results obtained at the end of this test showed that withdrawal to "Odontol" induced a significant decrease in the occurrence of immobility compared to the control mice and increases the total duration of immobility on day 24. These results are consistent with previous research that shown that ethanol-induced withdrawal rodent has depressive effects in the tail suspension test [33]. During the withdrawal period our results showed a significant decrease in the immobility time of withdrawn mice compared to the mice of the control group and a significant increase in the total duration of immobility at the end of the withdrawal (day 24). Studies using self-consumption of alcohol have reported that withdrawal increases depressive status in both forced swimming and tail suspension tests and that treatment with tricyclic antidepressants such as desipramine reduced the duration of immobility [34]. According to some authors, the repeated consumption of alcohol leads to brain neuro-adaptations that can lead to prolonged and permanent changes, especially at the level of the NMDA receptor subunits and their composition, which seem to persist during withdrawal [35]. The light-dark box test was developed by Crawley and Goodwin [24]. This paradigm is used to evaluate the anxiolytic effects of substances and plants in rodents. Mice have a natural tendency to avoid lighted areas. An increase in the time spent in the dark compartment and a decrease in latency are anxiety-like behaviors index [25,36]. Our results showed that repeated consumption of "Odontol" as well as withdrawal induced a significant decrease in the latency of mice compared to control mice. There has also been a significant decrease in the time spent in the illuminated compartment and an increase in the time spent in the dark compartment of the box. As evidenced by our results, withdrawal induced the development of anxiety related behaviours in animals. Indeed, as noted in previous research on the study of substances and drugs affecting the behaviour of animals, it was found that anxiolytics increased locomotion and time spent in the illuminated compartment while anxiogenes decreased them [37]. Similarly, mice placed in the clear compartment showed a reduced latency to pass in the dark compartment as well as increased time spent in the black section [38]. Studies that have used alcohol self-administration in their protocol to assess the anxious state of animals following alcohol withdrawal in this paradigm have found behavioural changes. Indeed, the animals treated with alcohol showed a marked reduction in the time spent in the light chamber compared to non-alcohol treated controls. These changes suggest that weaning increased anxiety in animals [39]. Excessive consumption of ethanol followed by withdrawal leads to withdrawal syndrome [40,41]. Anxiety is the most common and important feature of alcohol withdrawal. It is also considered the main negative motivation for relapse [42]. These signs and symptoms of alcohol withdrawal were attributed to central neurotransmitter disruption and ion channel activity. Evidence indicates that during ethanol withdrawal, there is an increase in the number of excitatory NMDA receptors [43] and a decrease in the number of GABA-A inhibitory receptors [44]. Therefore, a drug that facilitates the action of GABA or decreases glutamate activity may be effective in the treatment of withdrawal-induced anxiety [38].
5. Conclusions and Perspectives
- At the end of this study, which focused on the evaluation of the psychotropic effects induced by weaning with "Odontol" in the white mouse Mus musculus Swiss, it appears that long term consumption as well as withdrawal of "Odontol" resulted in an increase in the level of depression and anxiety, effects more marked at day 8 and beginning to escalate on day 24. Future studies will need to take us forward understanding the molecular base of these psychotic effects of “Odontol” withdrawal. They will precisely have to highlight hippocampal neuron density and activity, as neurotransmitters level evaluation, synaptic changes in Mossy fibres pathway including dendritic spine measurement and Long-term potentiation recording as well as Brain Derived Neurotrophic Factor messenger RNA level measurement in the dentate gyrus, CA1 and CA3 layers.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML