-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2018; 7(1): 1-5
doi:10.5923/j.neuroscience.20180701.01

Membrane and Its Fleet: The Vanguard of Memory
Mahmud Arif Pavel
The Scripps Research Institute, Jupiter, Florida, USA
Correspondence to: Mahmud Arif Pavel, The Scripps Research Institute, Jupiter, Florida, USA.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Memory, the information stored in living beings, is essential for any perception and action. It subserves many other functions that life would be impossible without it. However, the physical properties of memory at the molecular level is poorly understood. Here, the coding of memory has been delineated through the cell membrane and its associated molecules. Memories are described as ion movements (electricity, ion conductivity) through the microscopic changes of cellular components.
Keywords: Memory, Membrane, Ion channel
Cite this paper: Mahmud Arif Pavel, Membrane and Its Fleet: The Vanguard of Memory, Research in Neuroscience , Vol. 7 No. 1, 2018, pp. 1-5. doi: 10.5923/j.neuroscience.20180701.01.
Article Outline
1. Background
- Memory, a highly distributed process, is associated with all learning and behavioral activity [1, 2]. Although modern neuroscience sheds some light on this process by which the brain stores our daily experiences, understanding of the fundamental nature of memory is still limited. There is no consensus on how cognitive memory is encoded or stored in the brain [3]. There have been many suggestions concerning what changes in the neuron lead to the formation of memories. These changes should be structural or chemical at the molecular level in the cytosol or on the membrane [5]. The changes must initiate ion movement or chemical signals when it receives recalling cue in the form of electricity or chemistry [6]. Memory formation has been associated with the sprouting of new dendritic twigs which increase its connections upon activity [4]. The fact that old people have more branches associated with their neurons than younger people has been used as evidence that this increased branching of the cells allows for more connections which may help contribute to the brains overall capacity to form memories. Since chemical components (e.g., protein, lipid, carbs, etc.) are vital for every type of cell and have been implicated previously in memory function, it has also been suggested that memory could be coded among the chemicals of the neuron as well. The essence of all the current theories of memory formation is that a neuron which is not coded for memory initially can be coded for memory, and the memory would be stored as a result of activity. The wave of action potentials that sweep through the brain during each activity is sorted out and deposited in various sites of the brain [1]. However, it is yet to be explained how the waves of action potentials could change the molecules within the neurons to form a memory or help with the further recalling of the memory. The molecular mechanism of how memory is encoded, stored, and recalled from the senses by the brain system is still missing. Here, memory and its properties are being explained in terms of the membrane and the channels or proteins embedded in it.
2. Memory at Cellular and Molecular Level
- More than 10 billion nerve cells, interacting with each other, control both the internal and external activity of the body by regulating the firing of nerve impulses [7]. This firing involves the interplay of ions across the cell membrane of neurons which is the basis of all neural complexity [8]. At the core of this process are the ion channels which are the receptors that sense our surroundings, convert perceptions into the form of electricity, and then transduce the electricity to the brain for further processing that includes memory formation [6, 9]. The execution of memory, referring to when a memory is used for a particular action, is also accomplished through electricity. Bionic limbs are a good example of this phenomenon [10]. The memory of a certain movement is stored in the neurons of the brain. These neurons then give the muscle instructions on how to contract or move in the form of electricity that are conveyed through action potentials. When a muscle contracts in response to the action potentials, it produces small electrical signals which are detected by the electrodes of bionic limb. This electric signal is then used to operate and control this artificial limb. Therefore, the formation and modulation of the memory must be rooted in the temporal and spatial pattern of the electricity generated by the ion movements (ion conductivity) (Fig. 1a).A cell is composed of many molecules including protein, carbohydrate, nucleic acids and lipid. These chemical components therefore are likely to be involved in memory formation [11]. Although there is no direct proof yet, it is agreed that memory is encoded and stored using the language of electricity generated via cellular molecules [12]. The formation of memory is ingrained in the senses that begin with physical stimuli [13]. At the molecular level, all the senses are the result of ion movements which are initiated by the detections of particular stimuli associated with each individual sense [14, 15]. The ion movements are triggered by the ion channels or transporters (can be other types of proteins) when they detect their particular physical stimuli. This leads to the production of electrical signals that are carried by the neuron to gives rise to memory, behavioral responses, conscious perceptions, or other neural activity [16]. Therefore, ion channels, transported or similar protein that controls ion conductivity across the membrane can be coded for memory store per se or with different protein arrangement [17, 18] (Fig. 1a-b).
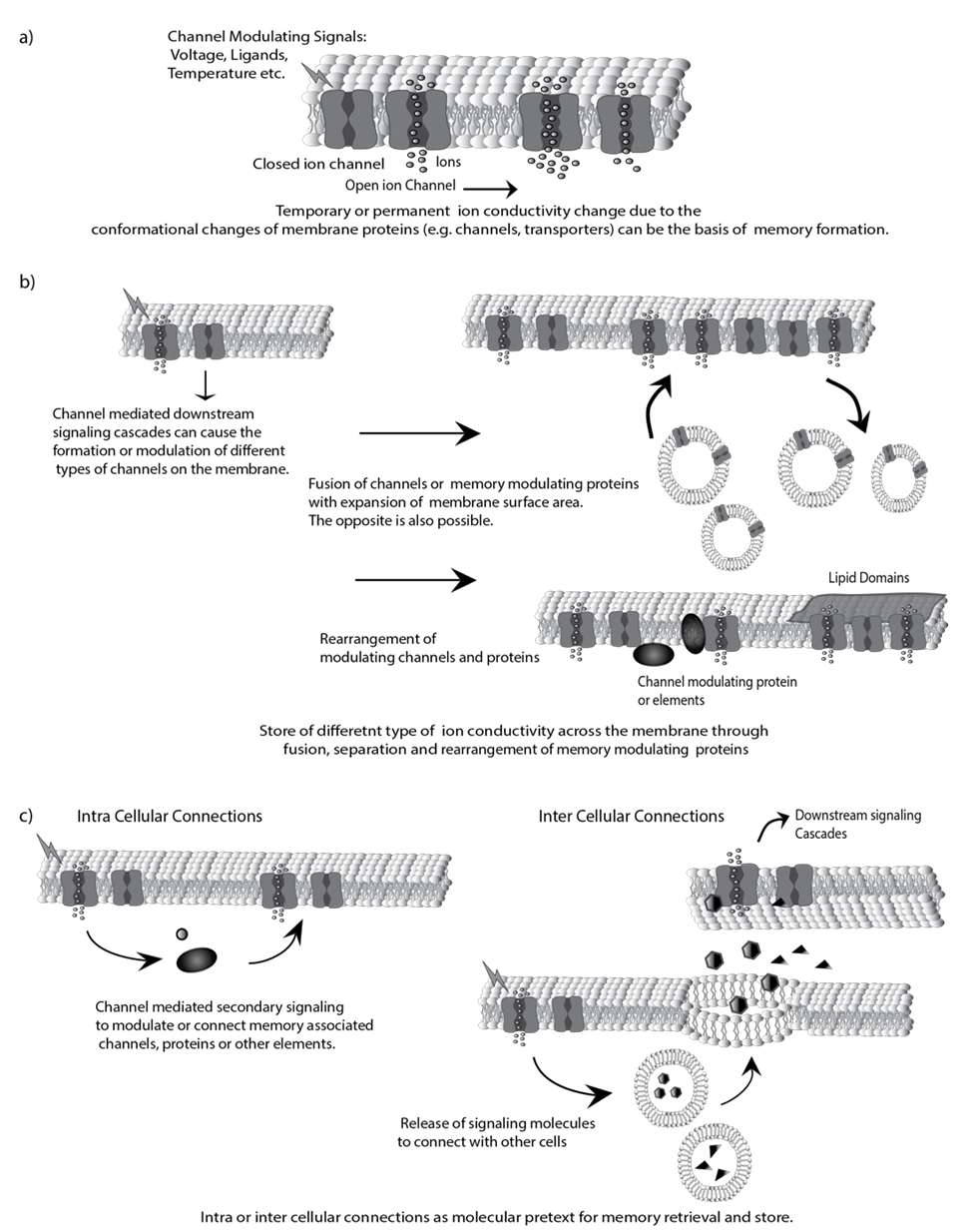 | Figure 1. Molecular basis of memory formation |
3. Membrane as the Basis of Memory
- All cells within the human body are enclosed in a lipid-made plasma membrane [19]. This membrane has a low intrinsic permeability to ions which can be overcome by the channels or transporters embedded in it [9]. For ion transport efficiency, channels have an advantage over carriers - they are about105 times faster than any known carrier protein [20]. Membranes and their embedded proteins allow neurons to receive, conduct, and transmit signals [21]. These principles may also serve as the basis for memory formation since ion channels can maintain specific ion movements (ion conductivity) across the membrane upon receiving a signal (Fig. 1a). This idea is analogous to microphone-speaker (Input-Output) systems found within computers. The microphone receives sound and converts it to electrical signals which are then stored in the computer in the form of charge which is converted through the use of transistors. When that sound is recalled, the computer reproduces the same electrical signals and the speaker translates these signals into an audible sound that is the same as the sound that was originally inputted. Living systems are also equipped with many input-outputs that solely rely on the electrical signals generated or modulated through the membranes associated ion channels and proteins. Upon receiving the corresponding signals, the properties of these channels or proteins can be altered and stored in a pattern such that they produce specific electrical signals every time they are recalled (Fig. 1). What is the measure of this electrical signals (ion conductivity)? There may not be any specific measurement since channels seem capable of differentiating any current change in the cell [22, 23]. A channel has the ability to change itself to provide a specific rate of ion movements or produce signaling cascades to store the ion conductivity dynamics across the membrane [5, 24-27] (Fig. 1a-b).When neurons conduct action potentials, they also generate heat and mechanical force [28, 29]. However, the mechanisms behind the production of thermal and mechanical waves in neuronal membrane associated with the generation of electrical impulses remains unclear. Recent studies have shown that mechanical force can disrupt the dynamics of lipid microdomains (lipid raft or lipid clusters) on the cell membrane [30, 31]. Therefore, it is tempting to predict that the mechanical waves on the membrane surface that are driven by the electrical wave produced by action potentials could alter the clustering properties of the lipids. A potential change in the lipid membrane due to action potentials has not yet been shown, but it would be very interesting to further investigate its potential role in memory formation and other neuronal activities. For example, as the electrical impulse travels, it could make mechanical imprints on the lipid membrane and contribute to the coding of memories and to the coordination of learning processes (Fig. 1b). The association of cellular membranes and other components of memory are becoming clearer with recent studies and further research. However, there is no experimental evidence so far as to whether any one or more of these chemicals could contribute to the storage of information deemed as memory.
4. Memory Characteristics Explained with Membrane Proteins
- Memory has many characteristics including recalling, remodeling and redundancy [32-34]. Membrane-associated proteins can form a mechanism to recall memory with specificity and accuracy (Fig. 1c). Memory forming, or modulatory ion channels, proteins or other elements can be arranged, localized or connected to different signaling to index and recall the memory. Memory is a collective change that occurs when an event causes neuron across the nerve and brain to fire (ion channels action). This action could alter gene expression and form or modify neuronal connections as a basis of memory formation and regulation. When a particular memory is recalled (through electrical signals i.e. the ion conductivity), these neurons fire back (similar ion channel action) and playback the brain activity associated with the past event. This idea corroborates the fact that when two persons recall the same event, similar brain imaging patterns are seen for the same memory. This observation and the corresponding similar brain activity suggests that memory is associated with cellular components (e.g. ion channels) and processes are conserved [35]. Additionally, when different persons recall the same memory but describe it differently, they can also have similar patterns of neural activity (similar ion channel action) [36]. Where are memories stored at the molecular level? Different regions of the brain process and interpret and process different sensations which they receive in the form of electrical signaling from variuous areas of the body. These sensations also triggers the recollection of memories for example stimulating the optical lobe creates visual sensations or the temporal lobe recalls specific experiences from the past [6, 37]. These electrical signals, renders to memories, are formed as changes are made by the wave of the nerve impulses s as they travel along their paths to various regions of the brain. Therefore, memories are somewhat localized through ion channel modification or arrangement in some area or all over the brain. If a memory could be found in a specific location in the brain, the elemental nature of the memory could be tested experimentally. There is valid evidence of memory localizations, and a memory can be pinned down to a specific set of memory-associated proteins [38, 39]. The process of memory begins with encoding, then proceeds to storage and, eventually retrieval. All these processes interact with and influence each other. For example, it has been observed that the recall of one memory can trigger another [7, 40]. Memory also changes over time [34, 40-42]. When people recall particular events, similar types of activity patterns appear throughout the brain. Membrane ion channels and other proteins are also altered and tuned over time [43, 44]. It appears that memories and the neurobiological mechanisms of the memory are linked. One memory is connected to others which helps in recollecting the memory. A clear connection has been established which demonstrates that repetitive activity can trigger memory formation and inactivity facilitates forgetting [3, 45]. This correlates with the fact that activity facilitates the passage of action potentials through neurons whereas inactivity hinders it. This process, also aids in making the memory system flexible [1, 46]. When a person stores or retrieves single memories, new and old memories can be connected, arranged, reshuffled or muddled. As memory is dynamic, memories are prone to remodeling and distortion. Interestingly, these qualities of memory could also provide a means to create imaginative and false memories [47]. Changes in ion conductivity due to the ion channel modulation, formation, separation, or rearrangement on the membrane can help explain how memories are arranged in the brain and connect to each other during the process of recalling, reshuffling, forgetting and creating false memories.The neuronal membrane can have multiple copies of memory associated channels and proteins (e.g., ion channel with specific conductivity that defines memory). Even the same pattern of channels and proteins can be present in various neurons. This complies with the fact that the memory formation is a highly-distributed process acting on multiple or whole parts of the brain. These memory associated channels and proteins are distributed across millions of neurons and each neuron can be associated with millions of memories. This creates redundancy and explains why extensive brain damage is usually not accompanied by a profound or widespread loss of past experiences [2, 48]. Memory associated channels and proteins create a collaborating pattern such that if part of the brain fails, the remaining will still suffice to represent past experiences.
5. Conclusions
- Memories are remembered, retrieved, recreated, remodeled, distorted and deleted. However further research will still be needed to find the exact mechanisms behind all the processes associated with the memory. Many questions remain to be answered on memory. For example, if memory is coded in the form of electricity through ion channels, then how it is coded? How does a channel differentiate various patterns of electricity? Indeed, it is difficult to answer these questions, and they have not been undertaken very seriously at a neuro molecular level even though the field of molecular biology is now exceedingly advanced. An understanding of the physical, chemical or electrical nature of memory would allow the researchers to better understand the complex features of memories and allow them to better be able to retrieve or modify memories experimentally. This could reveal, in the future, why memories can fade and even be completely forgotten in old age or with disease. Even today researchers have only revealed some of the basics of memory as it is very challenging to test the memory experimentally. However, over the past decade, new techniques for labelling and modifying specific neurons have emerged that could allow researchers to test how memories are formed and processed. Now is the realistic time to drive for it.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML