-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2017; 6(2): 28-34
doi:10.5923/j.neuroscience.20170602.02

Effect of Oral Administration of Some Selected Sweeteners on Anxiety in Wistar Rat Model
Suleiman Joseph Bagi1, Eze Ejike Daniel2, Karimah Mohammed Rabiu3, Iliya Ezekiel4, Sheu Oluwadare Sulaiman5
1Department of Science Laboratory Technology, Akanu Ibiam Federal Polytechnic, Unwana, Afikpo, Nigeria
2Department of Physiology, Faculty of Biomedical Sciences, Kampala International University, Kampala, Uganda
3Department of Biological Sciences, Faculty of Science, Yobe State University, Damaturu, Nigeria
4Department of Biological Sciences, Faculty of Pure and Applied Sciences, Federal University, Wukari, Nigeria
5Department of Physiology, Faculty of Medicine, Kampala International University, Dar es Salaam, Tanzania
Correspondence to: Eze Ejike Daniel, Department of Physiology, Faculty of Biomedical Sciences, Kampala International University, Kampala, Uganda.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Anxiety disorders represent one of the most common emotional diseases and consequently, the development of a suitable and lasting therapy is of important public health interest. This study evaluated the effect of oral administration of some selected sweeteners on anxiety in Wistar rats. Thirty-two (32) albino male Wistar rats were used. The rats were divided into eight (8) groups (GP1-8) of four rats each. GP1 rats served as control and were given water, GP2 rats were administered 0.05mg/kg b w of Diazepam, GP3 and GP4 rats received 100 and 200mg/kg b w of sucrose respectively, GP5 and GP6 rats received 100 and 200 mg/kg b w of saccharin respectively while GP7 and GP8 rats respectively received 100 and 200 mg/kg b w of honey. The Open Field Apparatus was used to obtain some parameters such as: Line Square Frequency, Center Square Frequency, Centre Square Duration, Stretch Attend Posture, Rearing, Grooming, Urination, Defecation and Freezing, by allowing the animal in the Open Field Apparatus for about five minutes. The result obtained showed that rats treated with 200mg/kg b w of honey (53.00±43.07) showed a statistically significant difference (p<0.05) in grooming when compared to negative control (31.00±6.00). In freezing, groups treated with 200 mg/kg b w of sucrose (29.75±7.13) and 100mg/kg b w of sucrose were statistically significant (p<0.05) (29.50±11.38) when compared with the negative control (20.00±2.09). It can be concluded that sucrose, saccharin and honey produced no significant harmful effects on the psychological and physiological disorders in the animals. However, sucrose, saccharin and honey administration, especially at a higher doses did produced an effect on anxiety as shown by few anxiety parameters tested.
Keywords: Open field, Anxiety, Honey, Sucrose, Saccharin
Cite this paper: Suleiman Joseph Bagi, Eze Ejike Daniel, Karimah Mohammed Rabiu, Iliya Ezekiel, Sheu Oluwadare Sulaiman, Effect of Oral Administration of Some Selected Sweeteners on Anxiety in Wistar Rat Model, Research in Neuroscience , Vol. 6 No. 2, 2017, pp. 28-34. doi: 10.5923/j.neuroscience.20170602.02.
1. Introduction
- Anxiety is a psychological and physiological disorder, characterized by fear, apprehension and poor concentration. The somatic manifestation of anxiety includes palpitation, tachycardia, tremor, sweating and hyperventilation [1-3]. The definition of fear and anxiety varies greatly. Anxiety is a future-oriented mood state associated with preparation for possible, upcoming negative events while fear is an alarm response to present or imminent danger (real or perceived). This view of human fear and anxiety is comparable to animal predatory imminence continuum [4, 5]. That is, anxiety corresponds to animal’s state during a potential predatory attack but fear corresponds to the animal’s state during predatory contact or imminent contact. The symptoms of anxiety include worry (verbal-subjection), avoidance (overt motor act) and muscle tension (somato-visceral activity) [6, 7]. Anxiety affects the body and mind, causing an organism to be under stress. The body releases a hormone called adrenaline, which prepares it for danger [8, 9]. This is what causes the physical symptoms such as sweating, pounding heart and rapid breathing [10]. Sweeteners are ingredients that add sweetness to food [11-13]. There are two types of sweeteners: nutritive and non-nutritive sweeteners. Examples of nutritive sweeteners are honey, sucrose and saccharin. Honey is a sweet and viscous fluid produced by honey bees (genus Apis) and other insects from the nectar of flowers. Honey is also a popular sweetener and grouped as a common household product used throughout the world [14]. Popularity comes not only from it being a natural sweetener but also from many proven or unproven benefits associated with it [15]. It has many medicinal uses described in traditional medicine. Modern system of medicine is also finding the honey efficacious in various medicinal and surgical conditions. Antimicrobial, antioxidant and wound healing properties of honey are being evaluated with successful outcomes. Prevention and treatment of various infections due to a wide variety of organisms and promoting surgical wound healing are some of the areas where honey is making its mark [16]. Orr et al [17], reported that 5% v/v concentration of honey decreased the duration of diarrhoea in cases of bacterial gastroenteritis.Saccharin has been the subject of extensive scientific research. It is one of the most studied ingredients in food supply. Although the totality of the available research indicates that saccharin is safe for human consumption, there has been controversy over its safety. The basis for the controversy rests primarily on findings of bladder tumors in some male albino Wistar rats fed with high doses of sodium saccharin [18]. Considerable saccharin research by U.S National Institutes of Health in 1997, however, indicated safety at human levels of consumption. In addition, the level of human consumption of saccharin is very small compared to the levels used in rat studies. Although past research found an increased risk of bladder tumors in male Wistar rats ingested high amount of saccharin, additional research has discovered that the mechanism by which the tumors developed was specific to male albino Wistar rats and does not apply in human [19].Sucrose is a sugar belonging to the group of carbohydrate known as disaccharides. It is soluble in water, slightly soluble in alcohol and ether, it crystallizes on long, slender needles, and is dextrorotary, (i.e. rotating the plane of polarized light to right) [20]. Sucrose is often extracted and refined from either cane or beet sugar for human consumption. Modern industrial sugar refinement processes often involve bleaching and crystallization to produce a white, odourless, crystalline powder with a sweet taste of pure sucrose that is devoid of vitamins and minerals [21]. This refined form of sucrose is commonly referred to as table sugar. It plays a central role as an additive in food production and food consumption all over the world [22]. Hence, this study was undertaken to evaluate the effect of oral administration of some selected sweeteners on anxiety in albino Wistar rats.
2. Materials and Method
- MaterialsSaccharin, honey and sucrose were purchased from Shoprite shopping Mall and Stores, Enugu State, Nigeria.Animal care Thirty-two (32) albino male Wistar rats were purchased from the Department of Veterinary Medicine, University of Nigeria, Nsukka, Enugu State, Nigeria and were housed in the Animal House of the Department of Science Laboratory Technology, Akanu Ibiam Federal Polytechnic Unwana, Afikpo Ebonyi State, Nigeria. They were fed with standard feed pellets and given water ad libitum.Open Field ApparatusThe Open Field Apparatus was constructed with white plywood, and consists of 72cm wide × 72cm long and 32cm high walls. One of the walls was made with Plexiglas, so that the rats could be visible in the apparatus. Blue lines were drawn with marker and were made visible through the Plexiglas floor. The lines divided the floor into sixteen 18cm x 18cm squares. A central square (18cm x 18cm) was drawn in the middle of the open field [23]. The central square was used because some rat strains have high locomotor activity and can cross the lines of test chamber many times during test session [24]. Also, the central square had a sufficient space surrounding it to give meaning to the central locomotion as being distinct from the outer locomotion.The following were measured: Line Crossing (frequency with which the rats crossed one of the grid lines with all four paws). Center Square Entries (frequency with which the rats crossed one of the red lines with all four paws into the central square). Center Square Duration (duration of time the rats spent in the central square). Freezing (duration with which the rat was completely stationary). Grooming (duration of time the rat spent licking or scratching itself while stationary). Defecation (number of fecal boli produced). Urination (number of puddles or streaks of urine). Stretch Attend Posture (frequency with which the rat demonstrated forward elongation of the head and shoulders followed by retraction to the original position). Rearing (frequency with which the rats stood on their hind legs in the maze). MethodsExperimental ProcedureThirty-two (32) albino male Wistar rats were used in this study. The rats were divided into eight (8) groups of four (4) albino Wistar rats each as follows:Group 1 was given only 0.5ml of water for 28 days. This group served as the control.Group 2 was given 0.05mg/kgbw of Diazepam.Group 3 was given 0.5ml of 100mg/kgbw of Sucrose for 28 days.Group 4 was given 0.5ml of 200mg/kgbw of Sucrose for 28 days.Group 5 was given 0.5ml of 100mg/kgbw of Saccharin for 28 days.Group 6 was given 0.5ml of 200mg/kgbw of Saccharin for 28 days.Group 7 was given 0.5ml of 100mg/kgbw of Honey for 28 days.Group 8 was given 0.5ml of 200mg/kgbw of Honey for 28 days.The behaviours of the animals on the first, second, third and fourth weeks were recorded. The behaviour recorded included the following: Line Square Crossing, Center Square Crossing, Centre Square Duration, Freezing, Grooming, number of boli of faeces, streaks of urine, Stretch Attend Posture and Rearing.Data analysisThe data obtained was expressed as mean ± SD of four determinations. The data analysis was done using one way analysis of variance (ANOVA) followed by Tukey’s post hoc test to determine the levels of significance between the control and experimental groups. All statistical analyses were evaluated using SPSS 20.0 and Microsoft Excel (2007). The values of P< 0.05 were considered significant.
3. Results
- Effect of Sweeteners on Anxiety in Wistar Rats for Week 1 Results obtained showed that there was a significant statistical difference (p<0.05) in Line Square Frequency for rats treated with 100mg/kg b w of saccharin (40.00±36.86) compared with the control (26.00±6.87). In the centre square frequency, rats treated with 200mg/kg b w of honey (1.50±0.70) showed a statistically significant difference) p<0.05) when compared with the control group (0.00±0.00). Similarly, the number of times the rats froze (13.00±9.89) was statistically significant (p<0.05) after administration of 100mg/kg b w of sucrose, when compared to the control (5.00±0.00). On the other hand, the number of grooms (27.25±11.05) was statistically significantly different (p<0.05) when treated with 200mg/kg b w of honey as compared to the control group (15.00±2.50).
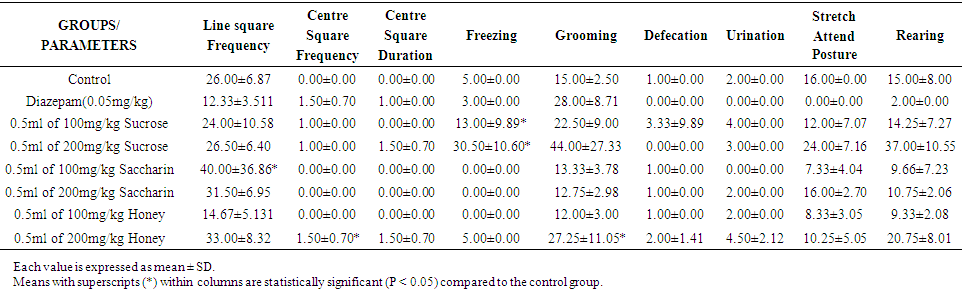 | Table 1. Effect of Sweeteners on Anxiety in Wistar Rats for Week 1 |
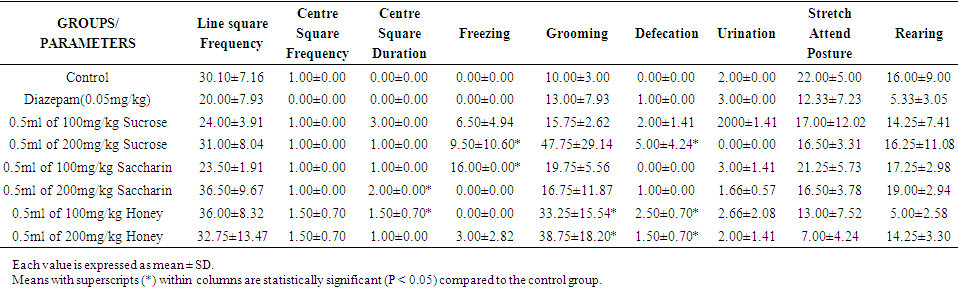 | Table 2. Effect of Sweeteners on Anxiety in Wistar Rats for Week 2 |
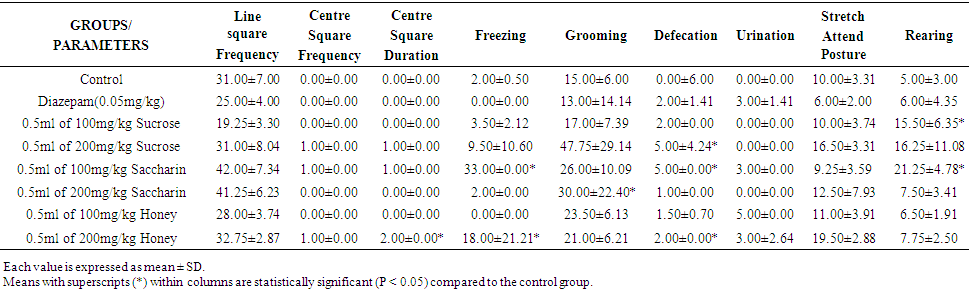 | Table 3. Effect of Sweeteners on Anxiety in Wistar Rats for Week 3 |
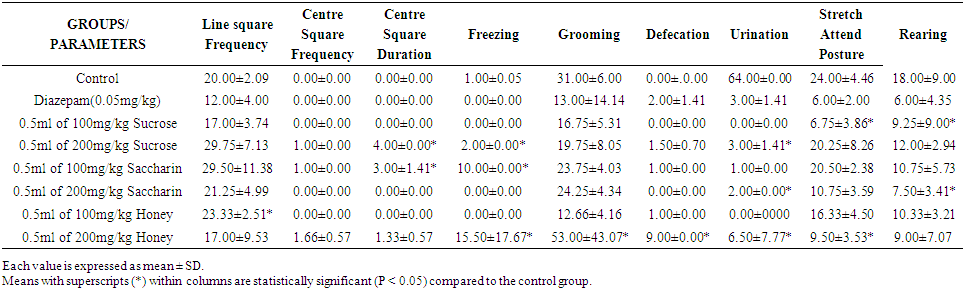 | Table 4. Effect of Sweeteners on Anxiety in Wistar Rats for Week 4 |
4. Discussion
- This research work was carried out to evaluate the effects of oral administration of some selected sweeteners (sucrose, saccharin and honey) on anxiety in Wistar rats. Open Field Apparatus was used to test for the anxiety to obtain some parameters such as Line Square Frequency, Center Square Frequency, Center Square Duration, Freezing, Grooming, Urination, Defecation, Stretch Attained Posture, and Rearing. There are limited studies on sucrose, saccharin and honey on anxiety. Therefore in this present study, the effect of some selected sweeteners on the brain was established using the open field test, to assess the level of anxiety in rats.The results obtained revealed that after four weeks of treatment (table 4), there was a statistical increase (p<0.05) in the Line Square Frequency in groups treated with 100mg/kg of saccharin (42.00±7.34) and 200mg/kg of saccharin (41.25±6.23) when compared with the control (31.00±7.00) but was statistically insignificant (P >0.05). Similarly, there were no values for the centre square frequency and centre square duration. This study then suggests that sucrose, saccharin and honey might not have interfered with some areas of the brain. The administration of sucrose, saccharin and honey in rats did not show placidity which is calmness with little or no response to provocation which might be as a result of the stimulation of the ventromedial nucleus of the hypothalamus [15]. Bilateral amygdaloid lesion has not also been seen to inhibit the rage area and facilitate placidity.Anxiety is a motor response to emotion and is controlled by complex mechanisms that involve association areas of the cerebral cortex, the limbic system and the hypothalamus [25]. The result obtained showed that the rate of fear (anxiety) reduced down the weeks. The albino Wistar rats were very much afraid in the week one. This caused them to freeze, become immobile and groom. However, in the week four, only groups treated with sucrose at high dose, sucrose at low dose and honey at high dose were afraid. In week one, the groups treated with sucrose at low dose, sucrose at high dose, low dose saccharin, low dose honey and high dose honey were observed to have psychological and physiological disorder associated with fear. In week four, only groups treated with high dose of sucrose, sucrose in low dose and honey in high dose had psychological and physiological disorder associated with fear. This might be due to low levels of GABA, a neurotransmitter that reduces activity in the central nervous system, contribute to anxiety [26]. A functional brain imaging techniques suggests that the effects of SSRIs in alleviating anxiety may result from a direct action on GABA neurons rather than as a secondary consequence of mood improvement [27].The lack of values for defecation and urination corroborates with studies of [28] which described defecation and urination as indices of anxiety in rodents. He argued that the animal will have reduced locomotion in a novel environment but the autonomic nervous system will be activated which will increase defecation in this noxious arena. However, [29] argued that there was no significant relation between fearfulness, urination and defecation as measured in the Open Field test. Nevertheless, Bindra and Thompson agreed that defecation and urination in a novel environment are signs of emotionality, which is not to be equated with fearfulness or timidity. A comparative study done on effect of ondansetron, granisetron and alprazolam on anxiety in Wistar rats by [30] showed that both alprazolam and ondansetron demonstrated anxiety which was evident from increase in time spent in the centre, as compared to periphery by the rats. It stated also that granisetron did not produce anxiety. However, in the present study, administration of sucrose, saccharin and honey especially at higher doses produced a significant effect on anxiety.
5. Conclusions
- The study has demonstrated that sucrose, saccharin and honey at doses administered in the current study, produced no significant harmful effect on the psychological and physiological disorders in the animals; however, sucrose, saccharin and honey administration, especially at a higher doses did produced an effect on anxiety as shown by few anxiety parameters tested.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML