-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2017; 6(2): 21-27
doi:10.5923/j.neuroscience.20170602.01

Assessment of Neurometals in Neonates of Preeclamptic Mothers
Samir Mohammed Mounir1, Mohamed Farouk Afify1, Hashem Faris Mohammed2, Mostafa Ahmed Elsayed3, Asmaa Nabil1
1Pediatrics Dept., Faculty of Medicine, Minia University, Egypt
2Obstetrics and Gynecology Dept., Faculty of Medicine, Minia University, Egypt
3Clinical Pathology Dept., Faculty of Medicine, Minia Univeristy, Egypt
Correspondence to: Samir Mohammed Mounir, Pediatrics Dept., Faculty of Medicine, Minia University, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Preeclampsia is a multisystem disease causing both maternal and fetal morbidity principally neurological tragedies. It may be associated with changes in concentrations of blood elements. We aim to assess neurometals; Selenium (Se), copper (Cu), zinc (Zn) and iron (Fe) concentrations in serum of 25 neonates of preeclamptic mothers and their mothers representing group (I) with comparison to 20 apparent healthy neonates and their healthy mothers representing group (II). Both groups were subdivided into two subgroups: a (Full Term) and b (Preterm). Results revealed that: Group (I) neonates had significantly (P < 0.01) lower Cu concentration than control (100.1 vs. 126.1 mg/dl) while, insignificant differences were observed between groups in serum Se, Zn and Fe concentrations. Higher levels of Zn, Cu and Fe were observed in full term neonates of preeclamptic mothers compared to preterm neonates of the same preeclamptic mothers. There was negative correlation between mean serum level of Se in preeclamptic mothers and weight of their neonates and also level of Cu of them was positively correlated with weight of their neonates. Positive correlation was found between mean serum level of Cu in preeclamptic mothers and that of their neonates. In conclusion, the studied neurometals may have influence on the health of pregnant women and fetus and their disturbance may contribute to pathogenesis of neurological complications in both mothers and fetuses. Screening status of these elements during pregnancy might be of value for correction of deficiencies with rationalized use to avoid dangerous excess with its neurological sequence.
Keywords: Neurometals, Neonates, Preeclampsia, Preterm, Full term
Cite this paper: Samir Mohammed Mounir, Mohamed Farouk Afify, Hashem Faris Mohammed, Mostafa Ahmed Elsayed, Asmaa Nabil, Assessment of Neurometals in Neonates of Preeclamptic Mothers, Research in Neuroscience , Vol. 6 No. 2, 2017, pp. 21-27. doi: 10.5923/j.neuroscience.20170602.01.
Article Outline
1. Introduction
- Preeclampsia has major contribution to prevalent vascular endothelial dysfunction and vasospasm occurring after the 20th gestational weeks and it is defined clinically by hypertension and proteinuria, with probable pathologic edema [1]. It may interrupt the balance between capillary and cellular perfusion pressures impairing the cerebral hemodynamics and other maternal neurological complications [2]. Preeclampsia is an initiate of eclampsia which is linked to life-threatening neurological complications like seizures, brain hemorrhage, strok, edema and brain herniation [3]. Pre-eclampsia can lead to fetal growth retardation, neonatal hypoxic ischemic encephalopathy and the sequelae of premature birth. Fetuses of preeclampic mothers develop slowly than normal ones due to reduction in nutrients and oxygen passed from the mother to her fetus [4].Due to its importance in the central nervous system function, some trace elements called neurometals [5] & [6] like Zinc (Zn), selenium (Se), copper (Cu) and iron (Fe). These neurometals concentrations are so critical to central nervous system metabolism rate and regeneration capacity [7].Zinc was linked to increased preeclampsia incidence and its supplementation reduced this high incidence [8]. Also, decreased Se and Cu levels have been observed in patients with preeclampsia [9]. Low Se level enhances preeclampsia by its effect on glutathione peroxidase (Gpx), an active enzyme in oxidative stress which reduces the formation of free radicals [10]. Zinc has an important role in growth, development, metabolism, and reproduction [11]. Copper (Cu) has been found to be an essential constituent of vital Cu-dependent enzymes acting as antioxidant defense system [12] and also, it is vital for embryonic development [13]. Iron is a necessary trace element, Fe-containing enzymes and proteins, participate in many biological processes [14].The aim of the current work is to assess selenium, copper, zinc and iron concentrations in serum of a group of neonates of preeclamptic mothers and their mothers in comparison with apparent healthy neonates and their healthy mothers.
2. Subjects and Methods
2.1. Subjects
- This work was a case control study conducted on 45 neonates and their mothers who were recruited from obstetric and neonatology departments in Minia University Hospital, Minia governorate, Egypt during the period from April 2014 to March 2015. The neonates and their mothers were classified into two groups: Group (I) Preeclamsia: Included 25 neonates and their preeclamptic mothers and this group was divided into two subgroups as follow: Group Ia (Full Term): Included 15 neonates (whose birth weight were ≥2500 g and gestational age were 37-40 week) and their preeclamptic mothers.Group Ib (Preterm): Included 10 neonates (whose birth weight were <2500 g or gestational age were <37 week) and their preeclamptic mothers.Group (II) Control: Included 20 apparent healthy neonates (with matched gestational age and sex) and their healthy mothers serve as control group and also they were divided into two subgroups as: Group IIa (Full Term): Included 10 full term neonates and their mothers.Group IIb (Preterm): Included 10 preterm neonates and their mothers.Preeclamptic mothers were diagnosed by hypertension, edema and albuminuria. Twins, neonates with congenital anomalies and hypoxia were excluded. Mothers with other diseases such as anemia, biliary cirrhosis, malignancy, hypothyroidism, rheumatoid arthritis, collagen disease and nephritic syndrome and chronic diseases such as diabetes, chronic and severe disorders in kidneys were excluded.
2.2. Study Variables
- Neonates and their mothers were subjected to:Medical and obstetric history taking from mothers: In terms of Name, age, residence, duration of marriage, consanguinity, previous history of preeclampsia, family history of preeclampsia, parity and previous deliveries, last menstrual period and expected date of delivery.Clinical examination of neonates: Physical examination includes assessment of anthropometric measurements (weight, height and head circumference), vital signs, limited vascular and neurologic examinations, and foot examination, APGAR score abdominal and chest examinations.Laboratory investigations: in terms of CBC, reticulocytes counts, liver function (ALT and AST), renal function (urea and creatinine) and Serum concentrations of Se, Cu, Zn and Fe. Colorimetric method was used for measuring Se, Cu, Zn and Fe concentrations according to Teitz [15].
2.3. Statistical Analysis
- Data was statistically analyzed using SPSS program (Statistical Package for Social Sciences) software version 20 for windows. Descriptive statistics were presented in mean ± standard deviation (SD), while for categorical data were presented in the form of frequency and percentage. Analyses between groups were done for quantitative variables using independent sample t-test. Multiple comparisons test (Duncan test, LSR) was used to get the significance among subgroups. Chi square (X2) test was used for qualitative data between groups. Correlation between variables was done by using Pearson's correlation coefficient. The significant difference was considered when probability value was < 0.05.
3. Results
3.1. Demographic Characteristics
- Regarding demographic data of studied mothers and their neonates, the results showed that there was insignificant difference between all mothers in preeclamptic and control groups regarding age, marriage duration, previous history of preeclampsia, gravidity, residence and family history of preeclampsia. Also, there were no significant differences were found between neonates of preeclamptic mothers and controls regarding sex, weight, length, head circumference, APGAR score and gestational age.
3.2. Mean Serum Neurometals Concentrations between Groups and Subgroups
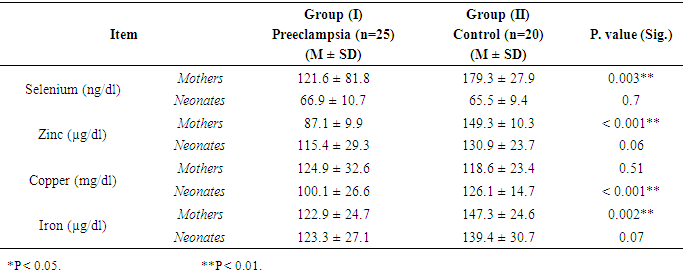
- The results presented in Table (1) revealed that preeclamptic mothers had significantly (P < 0.05 & P < 0.01) lower concentrations of serum Se, Zn and Fe than control ones, however insignificant difference was found in serum Cu concentration. In neonates, group (I) neonates had significantly (P < 0.01) lower Cu concentration than control (100.1 vs. 126.1 mg/dl) while, insignificant differences were observed between groups in serum Se, Zn and Fe concentrations.
|
|
3.3. Correlation between Mean Serum Neurometals Concentrations and Each of Weight and Gestational Age of Corresponding Neonate
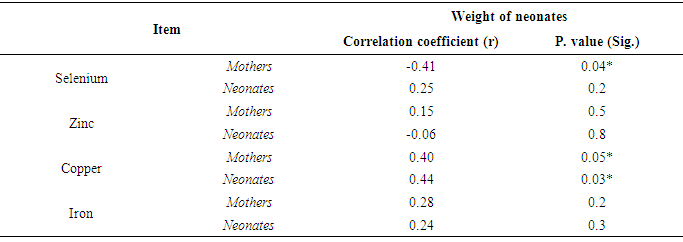
- Results in Table (3) showed that there was a significant negative correlation between mean serum level of Se of preeclamptic mothers and weight of their neonates (r = -0.41 & P <0.05). Also there was significant positive correlation between serum Cu of them and weight of their neonates (r = 0.40 & P <0.05). Whereas, there were no significant correlation between serum Zn and Fe of them and weight of their neonates. In neonates, the results revealed that there was a significant positive correlation between mean serum level of Cu of neonates of preeclamptic mothers and their weight (r = 0.44 & P < 0.05). While mean serum levels of Se, Zn and Fe of these neonates had insignificant correlation with their weight.
|
|
3.4. Correlation between Mean Serum Neurometals Concentrations of Mothers and Neonates
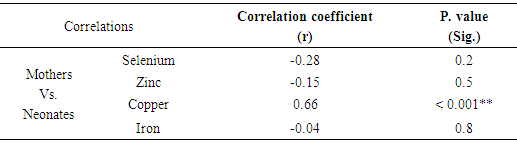
- The results showed that there was a significant positive correlation between serum Cu concentrations in preeclamptic mothers and Cu concentrations of their neonates (r =0.66 & P <0.05). While, no significant correlations were found in other elements (table 5).
|
4. Discussion
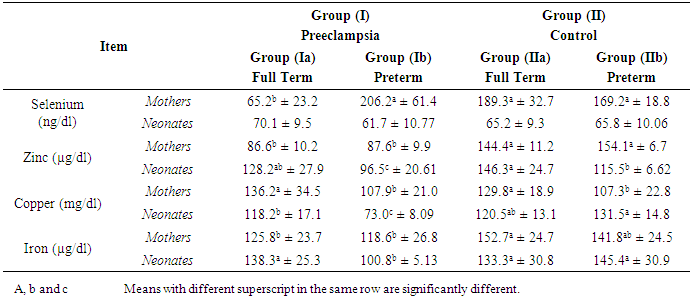
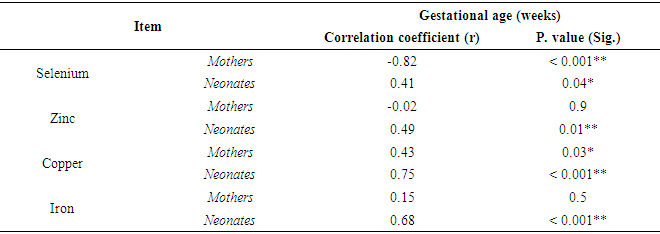
- Preeclampsia is a multisystem and multifactorial disease affecting both mother and her fetus by vascular dysfunction and by intrauterine growth restriction [16]. Some reports documented disturbance in blood trace elements concentrations in preeclampsia while others were contradictory [17]. Deficiency of trace elements in pregnancy is strictly related to mortality and morbidity in the neonate [18] also, can result in poor pregnancy outcomes [19] and the associated increased risk of adulthood diseases, including cardiovascular disease and type 2 diabetes [20]. The present study found significant lower levels of mean serum Se and Zn and Fe in preeclamptic mothers compared to control. Also our results showed that there was insignificant difference regarding mean serum level of Cu between preeclamptic and mothers controls. These results are in agreement with Rayman etal [21] & Akinloye et al [9] who found that there was a significant decrease in level of serum Se and Zn in preeclamptic mothers. Also, Farzin and Sajadi, [10] found a significant lower levels of serum Se and Zn in preeclamptic mothers compared to control. Similarly, Savita et al. [22] demonstrated that the serum iron decreased significantly (P<0.01) in preeclamptic pregnancy than normal pregnancy. The possible reasons of Cu alterations may be associated with the hormonal, metabolic and enzymatic variations in preeclampsia patients [23]. On the other hand, some authors reported a high zinc concentration in preeclamptic mothers compared to control mothers [24] & [25]. Whereas, Arash et al. [26] demonstrated that there was no significant difference in serum Zn between preeclamptic mothers and control.It was reported that deficiency in Se and Zn levels in pregnancy may cause disturbance of cellular antioxidant potential by reduction of superoxide dismutase activity, as well as increased lipid peroxidation leading to increase in blood pressure [27]. In preeclamptic mother, low serum zinc concentrations may be partly due to reduced concentrations of transport proteins and estrogen caused by increased lipid peroxidation. Also, the lowered serum concentrations of Zn have been suggested to be the result of reduced estrogen and Zn-binding protein concentrations [28].Our study showed significant lower level of serum Cu in neonates of preeclamptic compared to control groups. While, there was insignificant difference regarding mean levels of serum Se, Zn and Fe between all neonates of both groups. The present results are in accordance with those of Katz et al. [29] who found that there was significant lower level of Cu in fetal cord blood of preeclamptic group compared to control. However, this study found significant lower level of Se and significant higher level of Zn in fetal cord blood of preeclamptic group compared to control. Our results illustrated that there was insignificant difference regarding mean serum levels of Se, Zn, Cu and Fe between full term neonates of preeclamptic group and those of control group. To the best of our knowledge no studies are available concerning neurometalss in full term neonates of preeclamptic group compared with those of control group. We noticed that the difference in neurometals between full term groups and those of all groups and this may be due to maturity factor. So we recommended that maturity factor should be taken in consider in other studies.The present study found there was a significant lower level of serum Zn between preeclamptic mothers with preterm labour compared to those of control groups. Also showed insignificant difference regarding mean serum levels of Se, Cu and Fe between both groups of mothers. Similarly, Farzin and Sajadi [10] compared preeclamptic mothers with normal controls with mean gestational age of 35 weeks and found a significant lower levels of serum Se and Zn in preeclamptic mothers compared to control. In the current study, significant lower levels of serum Zn, Cu and Fe were found in preterm neonates of preeclamptic mothers compared to those of control group. Also there was insignificant difference regarding mean serum level of Se between both groups of neonates. Confirming our results, the study by Dawson et al. [30] who reported lower amniotic fluid Se concentrations in 29 preeclamptics delivering between 33 and 36 weeks gestation compared to 48 gestation matched controls and also reported increased amniotic fluid Cu concentration from 19 preeclamptic women compared to 53 controls. Also, they reported that amniotic fluid Zn concentrations decreased in preeclamptic women delivering preterm (33- to 36-weeks gestation).It was found that mothers of preterm infants did not differ in mean serum Zn or Cu concentrations from mothers with full term infants [31]. Also, there were no differences with gestational age either in maternal plasma Zn or Cu [32]. During pregnancy plasma Cu concentrations significantly increase returning to normal nonpregnant values after delivery [33] and [34]. Also the same study found that plasma Zn concentrations decline as pregnancy progresses. Ali et al. [35] found that significant lower difference between mean serum Se levels in maternal blood of full term and preterm neonates.Also our study showed that there were significant lower levels of serum Zn, Cu and Fe in preterm neonates of preeclamptic mothers compared to full term neonates of preeclamptic group. While there was insignificant difference regarding level of serum Se between these neonates. Broa et al. [31] found that preterm infants had significantly lower serum Cu concentrations than normal infants (p =0.01), whereas there was no difference in serum Zn concentrations between them. But, Perveen et al. [32] found that cord plasma Zn decreased with gestational age at birth and the reverse was observed for Cu. Significant difference between serum Se level in umbilical cord blood of full term and preterm neonates [35].Our study showed that there was significant negative correlation between mean serum level of Se of all preeclamptic mothers and weight of their neonates. Also there was significant positive correlation between mean serum level of Cu of all preeclamptic mothers and weight of their neonates. While mean serum levels of Zn and Fe of all preeclamptic mothers had insignificant correlation with weight of their neonates. These results are in agreement with those found by Ozdemir et al. [36] who found insignificant correlation between maternal serum Zn and Fe and birth weight and also identified that there was significant negative correlation between Cu level in maternal blood and birth weight. Mistry et al. [37] found lower maternal plasma selenium concentrations on 28 mothers who delivered SGA babies compared to 143 healthy controls. Lorena [38] identified that there was insignificant correlation between levels of Se, Zn and Fe in maternal blood and birth weight and identified that there was significant negative correlation between Cu level in maternal blood and birth weight. Also our study demonstrated that there was significant positive correlation between mean serum level of Cu of all neonates of preeclamptic mothers and weight of these neonates. Also mean serum levels of Se, Zn and Fe of all neonates of preeclamptic mothers had insignificant correlation with weight of these neonates. Placental zinc values of preeclamptic mothers positively correlating with birth weights [39]. Additionally, there was strong positive correlation between cord serum Zn and birth weight in SGA neonates but no correlation were found between cord serum Zn level and birth weight in AGA. The same study found insignificant correlation between cord serum Cu and birth weight in SGA and AGA [40]. There was insignificant correlation between Se, Cu and Fe in cord blood and birth weight and found also negative correlation between Cu level in cord blood and birth weight [38]. The present study reported that there was significant negative correlation between mean serum level of Se in all preeclamptic mothers and gestational age of their neonatesand there was significant positive correlation between mean serum level Cu of them and gestational age. Also there was insignificant correlation between mean serum levels of Zn and Fe of them and gestational age. Shand et al. [41] found that there was insignificant correlation between mean serum levels of Zn, Cu and Fe in preeclamptic mothers and gestational age. However, mean serum level of Zn in preeclamptic mothers positively correlated with gestational age and mean serum level of Cu was negatively correlated with gestational age but insignificant correlation [42].Also, our study reported that there was significant positive correlation between mean serum levels of Se, Zn, Cu and Fe in all neonates of preeclamptic mothers and gestational age of them. In a study by Srivastava et al [18] observed weak significant correlations between gestational age of the baby and Fe (r =0.23; p <0.05) and Cu (r =0.31; p<0.05) levels in the cord blood. Furthermore, we found that there was significant positive correlation between mean serum level of Cu in all preeclamptic mothers and that of their neonates, while there was insignificant correlation between mean serum levels of Se, Zn and Fe in preeclamptic mothers and those of their neonate. Zn and Cu in maternal and umbilical cord blood in normal pregnants had positive correlation [43].From our study limitations, limited number of cases as we included mothers and neonates and each of them subdivided into subgroups of full term and preterm. Also, mothers with preeclampsia who were included in our study were in moderate degree of the disease as mothers with severe preeclampsia were complicated by eclamptic fits and intrauterine fetal death. Also some of mothers were not cooperative enough. In conclusion, neurometals have important influence on the health of pregnant women and growing fetus. Our results revealed that mean serum concentrations of Se, Zn and Fe decreased in preeclamptic mothers and this reduction of these elements might be related to the etiology of preeclampsia. Neonates of preeclamptic mothers had low levels of serum Cu. Also, mean serum Se, Zn and Fe levels were lower in preeclamptic mothers of full term neonate than healthy mothers with matched gestational age. We recommend that all pregnant women should be screened for neurometals status for correction of deficiencies with rationalized use to avoid dangerous excess. We hope that our results could help to the knowledge of the relation between neurometals and preeclampsia and further studies should be conducted on studying this relation.
ACKNOWLEDGEMENTS
- The authors acknowledged and thank all volunteers who participated in this study and the clinical staff involved in the conduct of this study.
Ethical Standards
- Data were collected as part of a clinical study performed in obstetric and neonatology departments in Minia University Hospital, Minia governorate, Egypt. The study was approved by the Research ethics committee of faculty of medicine, Minia University, Egypt.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML