-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2017; 6(1): 11-20
doi:10.5923/j.neuroscience.20170601.03

Intra-Amygdala Injection of 5-HT1A Receptor Ligands Produces Differential Effects on the Behavior of High- and Low-Anxiety Wistar Rats
Maria P. Rysakova, Irina V. Pavlova, Nadezhda D. Broshevitskaya
Department of Conditioned Reflexes and Physiology of Emotion, Institute of Higher Nervous Activity and Neurophysiology of RAS, Moscow, Russia
Correspondence to: Maria P. Rysakova, Department of Conditioned Reflexes and Physiology of Emotion, Institute of Higher Nervous Activity and Neurophysiology of RAS, Moscow, Russia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the present study, the effects of intra-amygdala injection of an agonist (8-OH-DPAT, 0.3 µg/0.5 µL) and an antagonist (WAY-100635, 0.2 µg/0.5 µL) of 5-HT1A receptors on the behavior of high- (HA) and low-anxiety (LA) rats were compared. Administration of 8-OH-DPAT caused anxiolytic- and panicolytic-like effects only in LA rats; it increased the time spent in the open arms of the elevated plus-maze (EPM) and latency to leave the open arm of elevated T-maze (ETM). Administration of WAY-100635 caused anxiogenic effect in HA rats; it decreased the percent of open arms entries and increased the latency to the first enter an open arm in EPM. In LA rats, injection of the antagonist caused weak anxiogenic effect in the EPM and increased the locomotor activity. These results suggest that LA rats were more susceptible to activation of 5-НТ1А receptors in basolateral amygdala than HA animals. It is presumed that the functional activity of 5-НТ1А receptors in amygdala of low-anxiety animals is higher.
Keywords: High-anxiety and Low-anxiety rats, 5-НТ1А receptor, Amygdala, Elevated plus-maze, Elevated T-maze
Cite this paper: Maria P. Rysakova, Irina V. Pavlova, Nadezhda D. Broshevitskaya, Intra-Amygdala Injection of 5-HT1A Receptor Ligands Produces Differential Effects on the Behavior of High- and Low-Anxiety Wistar Rats, Research in Neuroscience , Vol. 6 No. 1, 2017, pp. 11-20. doi: 10.5923/j.neuroscience.20170601.03.
Article Outline
1. Introduction
- Anxiety is a negative emotional state of perception of potential or ambiguous threat [9]. In animals and humans, the transient condition of anxiety-related behaviors (state anxiety), that is only observable at particular moments, is distinguished from an affective characteristics of an individual that is the relatively permanent and stable across time (trait anxiety) [11]. In animal populations, there are individuals with different levels of anxiety, which has allowed for genetic breeding of animals with high and low anxious phenotype [11, 16]. Neurophysiological and neuromediator mechanisms defining the differences in the level of anxiety of animals and humans represent an important and still not fully understood problem. Search for the mechanisms is of particularly great importance for clinics, since humans may develop pathological anxiety disorders, such as panic attacks, phobia, and generalized anxiety disorders [36]. The probability of developing and efficiency of treatment of anxiety disorders, as well as the effects of some anxiolytic agents, have been shown to be individual-based [4, 19, 24, 37].Serotonin (5-hydroxytryptamine; 5-HT) is known to be involved in regulation of anxiety, fear and panic-related behavior [13, 22, 46]. Impairment of the serotoninergic system functions is associated with the development of various anxiety disorders, depressions, and other pathologies [33]. One of the most extensively distributed 5-HT receptors is 5-HT1A. The 5-HT1A belongs to the family of membranotropic Gi/Go protein-coupled receptors and mediates inhibitory neurotransmission [26, 33]. In the brain, 5-HT1A receptors are expressed as both an autoreceptors on cells in the dorsal and median raphe nuclei and a heteroreceptors, in forebrain regions involved in regulation of emotions [2, 33]. The 5-HT1A receptors are highly enriched in the amygdala, which is one of the key structures involved in anxiety regulation [42]. It is proposed that basolateral (BLA) nuclei serves as a major integrator and relay center for the sensory information necessary for anxiety, whereas the central (CeA) is the main output for the autonomic and somatic components of fear reaction through major projections to other limbic regions [20]. The amygdala receives the 5-HT innervations from the dorsal raphe nucleus [2].The 5-HT1A receptors play an important role in regulation of anxiety. The 5-HT1A receptor gene knockout mice demonstrated increased anxiety [29, 41]. Activation of 5-HT1A receptors in amygdala produces anxiolytic and panicolytic effects on the behavior of animals in T-maze, light–dark box, Vogel conflict test, and social interaction test [18, 39, 47]. The effects of some anxiolytic agents used in clinics are mediated by 5-HT1A receptors [26]. The binding potential of selective 5-HT1A receptor radioligand ([carbonyl-11C]WAY) is lower in amygdala, some areas of cortex, and raphe nucleus in patients suffering from social anxiety disorders [17].In addition to 5-HT1A receptors, 5-HT2А/2С and 5-HT3 receptors have been found in the amygdala. In contrast, their activation produces anxiogenic effect [1, 5, 8, 44]. The same subtype of 5-HT receptors can exist on both glutamatergic and GABAergic cell types of the amygdala; besides, different subtypes of 5-HT receptors can be located on the same neuron [2]. Thus, 5-HT in amygdala regulates the anxiety in complex manner. Based on the data on importance of amygdalar 5-HT1A receptor in regulation of anxiety, we hypothesized that 5-HT1A receptors of the amygdala, along with other receptors (D1, D2, GABAA etc.) could be involved in mechanism underlying the different anxious phenotypes [30, 35]. In the literature there are data that the binding potential of selective 5-HT1A receptor radioligand ([carbonyl-11C]WAY) negatively correlates with the level of anxiety in healthy patients [40]. It was shown that the animals with different duration of avoidance of the open arms of elevated T-maze differ in their sensitivity to systemic injections of 5-HT1A receptors agonist 8-OH-DPAT and antagonist WAY-100635 [43]. It was shown that the basolateral amygdala infusions of 8-OH-DPAT were not able to change the fear-potentiated startle in rats with high unconditioned startle response amplitude, but were able to in animals presenting low startle amplitude [10]. To our knowledge, studies concerning the influence of intra-amygdala microinjections of 5-HT1A agonist and antagonist on the behavior in different animal models of anxiety and panic in rats with different anxious phenotypes are lacking. However, such studies can provide new facts for better understanding of the mechanisms underlying individual differences in sensitivity to serotonergic agents in humans [15, 24]. The aim of the current study was to investigate the role of 5-HT1A receptors of the basolateral amygdala in regulation of anxiety in animals with high and low anxiety levels. The work includes the analysis of the behavior of high- and low-anxiety rats in animal models of anxiety and panic upon microinjection of an agonist (8-OH-DPAT) and an antagonist (WAY-100635) of 5-HT1A receptors to the amygdalar basolateral nucelus. Animal behavior was tested in the elevated plus-maze which is widely used for anxiety studies [11]. Thus, to analyze the animal behavior, we also used the elevated T-maze, which, along with anxiety, evaluates panic-like behavior [46], which is also the subject of regulation by 5-HT1A receptors.
2. Methods
2.1. Subjects
- Sixty-one male Wistar rats (350–450 g) were obtained from the campus of Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency, Russia. Rats were housed in a temperature-controlled vivarium (22°C ± 2°C) under a 12-h light/dark cycle (lights on at 08.00 h) with food and water ad libitum. Animals were kept 5 per cage before surgery and individually, after cannula implantation. Animals were handled daily for 3–4 days before experiments. All experimental procedures were conducted in accordance with Council Directive 2010/63EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes. The study protocol was approved by the Ethics Committee of the Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Sciences. Enough animals were used to ensure reliability of the result and every effort was made to minimize animal suffering.
2.2. Behavioral Testing
- Elevated plus-maze (EPM) was made of black-colored wood and consisted of two opposing open arms (OA) (10 × 50 cm each, with 2-mm rims) perpendicular to two opposing enclosed arms (EA) (10 × 50 cm each, with 40-cm side walls), all joined in the central area measuring 10 × 10 cm. The EA had no end walls. The maze was elevated to a height of 50 cm from the floor. The level of illumination was 150 and 90 lux on the floor level in open and enclosed arms, respectively (Digital Lux Meter AR823+, Smart Sensor). Each rat was placed in the middle of the central area facing the open arm and allowed 5 min of free exploration. The maze was thoroughly cleaned with 70% EtOH solution between rats. The behavioral parameters were scored as follows: number of open arm entries, time spent in the open arms, the latency to first enter an open arm, number of enclosed arm entries, distance moved, duration of moving (%), velocity, number of rearing, number of head dipping (looking down over the edges of the EPM), number of looking out of enclosed arms (investigative behavior performed in enclosed arms and directed towards open arms), number of stretch attend postures (forward extension of head and shoulders followed by retraction to the original position), duration and frequency of self-grooming, number of fecal boli and urination frequency. For each animal, the percentage of open arm entries (100 × open/(open + enclosed)) and the percentage of time spent in the open arms (100 × open/(open + enclosed)) were calculated. Elevated T-maze (ETM) was made of wood and had three arms of equal dimensions (10 × 50 cm each). One of the arms, enclosed by 40-cm high walls, was perpendicular to two opposed open arms surrounded by 2-mm rims. One day before the test, rats were exposed to one of the open arms of the T-maze for 20 min. A wooden barrier mounted on the border between the maze central area and the proximal end of the open arm isolated this arm from the rest of the maze. On the test day each animal was initially placed at the distal end of the closed arm of the elevated T-maze facing the intersection of the arms in order to measure inhibitory avoidance of the open arm. The time taken by the rat to leave this arm with four paws was recorded (Trial 1). The same measurement was repeated in two subsequent trials (2 and 3) at 30-s intervals. Following avoidance training (30 s), rats were placed at the end of the same previously experienced open arm and the latency to leave this arm with four paws was recorded 3 consecutive times with 30-s inter-trial intervals (one-way escape). The apparatus was cleaned with 70% ethanol after each animal and allowed to dry before the next subject was tested. A cutoff time of 300 s was established for the avoidance and escape latencies.An “EthoVision 3.1” (Noldus Information Technology b.v., 2003) integrated system was used for automatic recording and analysis of movement and behavior. Additionally, behavior in all behavior tests was recorded by a video camera linked to a monitor.
2.3. Selection of High-(HA) and Low- (LA) Anxiety Animals
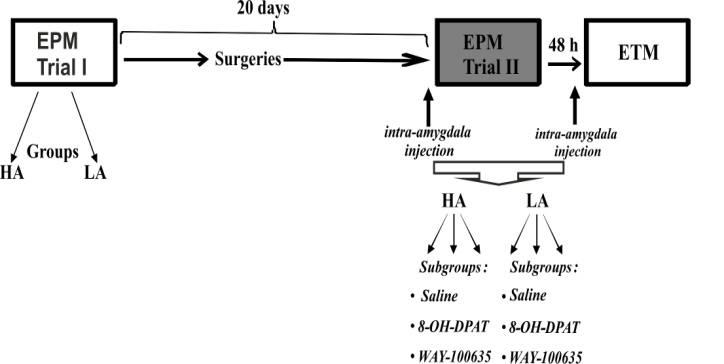
- In the present work, in order to assess appropriately the influence of 5-HT1A ligands in high- and low-anxiety animals, rats were split into groups (HA and LA) based on the percentage of the time spent in the open arms of EPM (Fig. 1).After the first trial in EPM (Trial I), rats were sorted by the percentage of the time spent in the open arms and split at the median (15.52%) into HA (% open arm time below median, n = 30) and LA (% open arm time above median, n = 31) rats [3, 27].
2.4. Stereotaxic Surgery and Microinjections
- About 7–9 days after the Trial I in EPM and 8–11 days before microinjection procedure, rats were anesthetized intraperitoneally with chloral hydrate (400 mg/kg), and restrained in a stereotaxis, and the incisor bar was adjusted so that the heights of lambda and bregma were equal. The stainless steel guide cannula (23 gauge) with injection cannula (30 gauge, dental needle, Ni-pro) were implanted bilaterally in basolateral amygdala according to rat brain atlas [32]. Stereotaxic coordinates for cannula placement were: −2.8 mm posterior to bregma, ± 4.8 mm lateral to the sagittal suture, and 8.5 mm ventral to bregma. The guide cannula was fixed to the skull with acrylic dental plastic. The tip of injection cannula was 1 mm below of the guide cannula. The injection cannula was taken out and dummy cannula (30 gauge, dental needle, Ni-pro) was inserted in the guides to prevent contamination.Both HA and LA rats were split into three equal subgroups (Fig. 1): one subgroup received saline (control, 0.5 µL), two other subgroups received 5-HT1A receptor agonist (8-OH-DPAT, 0.3 µg/0.5 µL, Sigma-Aldrich) or antagonist (WAY-100635, 0.2 µg/0.5 µL, Sigma-Aldrich). The drugs were dissolved in 0.9% NaCl and injected bilaterally into basolateral amygdala. The doses of drugs used were selected based on the literature data [10, 12, 39]. A volume of 0.5 µL of saline, 8-OH-DPAT, or WAY-100635 was delivered at a rate of 0.25 µL/min through an injection cannula connected with a polyethylene tubing to a 10-µL Hamilton syringe mounted on an Integrated Stereotaxic Injector (Stoelting Co, USA). After infusion, the cannula was left in place for an additional minute to allow diffusion of the solution and to reduce the possibility of reflux. Rats were tested in the EPM (Trial II) and ETM 10 min after the injections. The intervals between different tests were at least 48 h. The average interval between Trial I and II in EPM was about 20 days (Fig. 1).
2.5. Histology
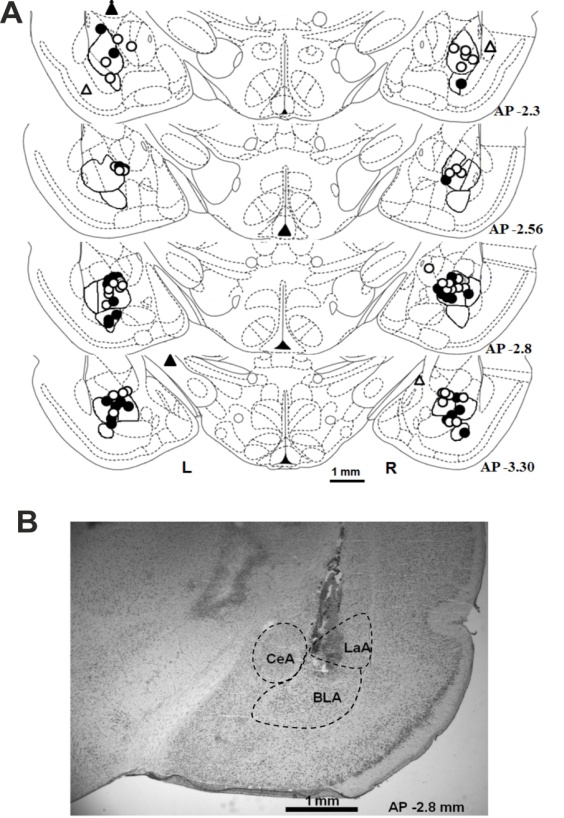
- Upon the completion of the behavioral experiments, animals were deeply anaesthetized with intraperitoneal overdose of chloral hydrate (1000 mg/kg). The animals were decapitated 10–20 min after the injection, brains were removed and maintained in 10% formalin solution for 2–3 days. Serial 180 μm coronal brain sections were cut with a freezing microtome. Cannulae placements were reconstructed on stereotaxic atlas templates from Paxinos and Watson (Fig. 2) [32].
2.6. Data Analysis
- Statistical analysis was performed using Statistica 8.0 (StatSoft). The data are presented as means ± SEM. The Basic Statistics was used to calculate the median percentage of the time spent in the open arms of EPM. Mann–Whitney U-test was used to compare the behavior of HA and LA rats during the first trial in EPM. One-Way ANOVA was used to compare the behavior of subgroups of LA and HA rats during the first trial in EPM. Two-way repeated measures ANOVA was used to assess the effects of saline, 5-HT1A agonist, and antagonist on the behavior of HA and LA rats in EPM. The Drug and Group were as between-subjects factors, Trial was as within-subject factor. Factorial ANOVA with three factors (Drug, Group and Trial) and two factors (Drug and Group) were used to analyze behavior data in the ETM. The main effect or interaction was followed by post-hoc comparisons using the Fisher's Least Significant Difference (LSD) test. A probability level of less than 0.05 was considered statistically significant. The probability level 0.05 ≤ p ≤ 0.1 was considered as tendency towards behavioral changes.
3. Results
3.1. Morphological Control
- Sixty-one rats were subjected to the surgery of cannula implantation. Of these 61 rats, 6 died before testing because of postoperative complications. Fifty-five implanted rats were tested in EPM (Trial II). Of these 55 rats, 36 rats were tested in ETM. The verification of cannulae placements in the brain of fifty-five tested rats (Fig. 2) revealed that tips of 87 cannulas were located in the basolateral nucleus of amygdala, 13 were at the border between the central and basolateral nuclei, three, in central nuclei, one, in lateral nucleus, one, in basomedial nucleus, and five, out of the amygdala nuclei. Only the data from rats with at least one cannula terminated in the basolateral amygdala were included in the analysis. No differences were found between the cannula tip locations between HA- and LA-animals.
3.2. Behavior of High- and Low-anxiety Rats
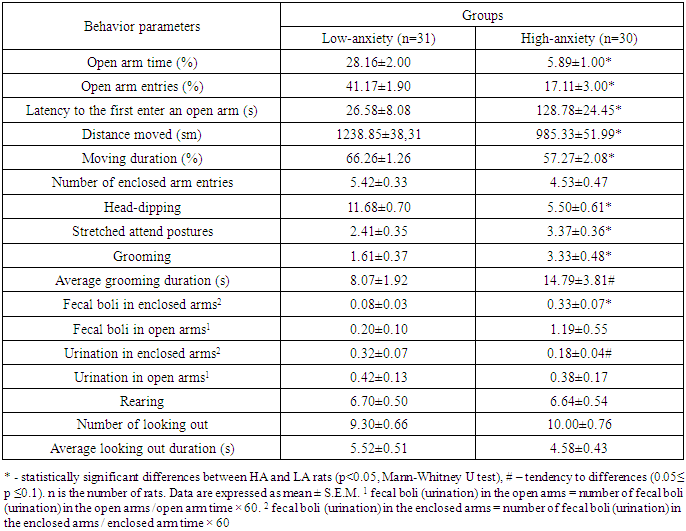
- The behavioral parameters of HA and LA rats grouped by the percentage of time spent in the EPM open arms during the first trial are reported in Table 1.
|
3.3. The Effect of 5- НТ1А Receptor Agonist and Antagonist Injection into the Amygdala on the Behavior of Low- and High-Anxiety Rats
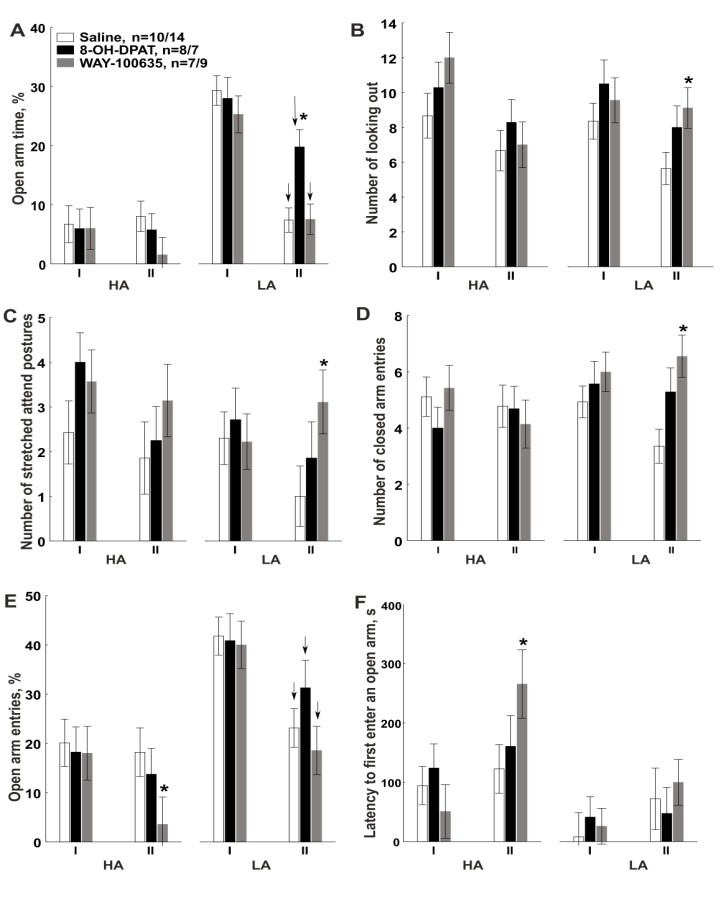
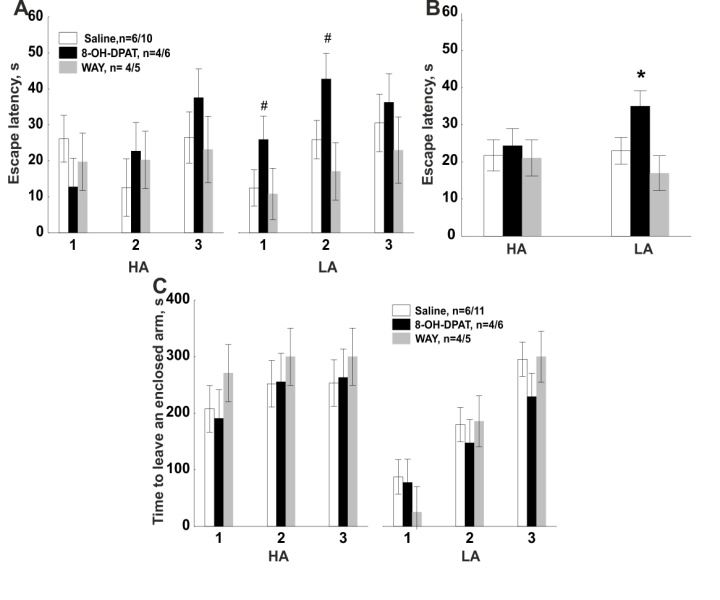
- Elevated plus-maze. Two-way repeated ANOVA revealed effect of Trial in EPM for the percentage of open arm time (Trial Effect: F1.48=35.89, P=0.0001, Fig. 3А), the percentage of open arm entries (Trial Effect: F1.48=27.68, P=0.0001, Fig. 3 E), the number of looking out of enclosed arms (Trial Effect: F1.48=11.14, P=0.002, Fig. 3B), rearing (Trial Effect: F1.48=9.3, P=0.004), the number of head dipping (Trial Effect: F1.48=85.47, P=0.0001) and the latency to first enter an open arm (Trial Effect: F1.48=8.22, P=0.006, Fig. 3 F). However, the percentage of open arm time (Fig. 3 A), open arm entries (Fig. 3 E) and number of head dipping decreased on the second trial mostly in LA rats (Group × Trial interaction: F1.48=27.16, P=0.0001, F1.48=4.64, P=0.036, F1.48=15.09, P=0.0001, respectively). Drug × Trial × Group interaction (a tendency) has been shown for the percentage of open arm time (F2.48=2.68, P=0.079). Repeated ANOVA did not revealed the main effect of Drug for any behavior parameters. One-way ANOVA revealed that on the first trial there were no significant differences between subgroups of HA and LA rats that received different agents on the second trial (Fig. 3A-F). Post-hoc comparisons revealed that on the second trial, significantly higher the percentage of time spent in the open arms was observed in LA rats after intra-amygdala injection of the agonist 8-ОН-DPAT compared to the control (Fig. 3A; P=0.002, Fisher LSD). No other behavioral parameter of LA rats was affected by 8-ОН-DPAT (Figs. 3B–F, P>0.05, Fisher LSD). 8-OH-DPAT injection in the amygdala did not influence the behavior of HA rats in the EPM (P>0.05, Fisher LSD).Post-hoc comparisons revealed that WAY-100635 injection decreased the percentage of open arm entries (P=0.049, Fisher LSD, Fig. 3E), increased the latency to first enter an open arm (P=0.004, Fisher LSD, Fig. 3F) compared to the control in HA rats. Post-hoc test revealed that injection of an antagonist increased number of looking out of enclosed arms (P=0.029, Fisher LSD, Fig. 3B), number of stretch attend postures (P=0.025, Fisher LSD, Fig. 3C) and number of enclosed arm entries compared to the control (P=0.009, Fisher LSD, Fig. 3D) in LA rats. Therefore, injection of 8-ОН-DPAT caused anxiolytic-like effect only in LA rats, but behavior of HA animals was not affected by an intra-amygdala injection of 8-ОН-DPAT. Injection of WAY-100635 caused anxiogenic-like effect in both LA and HA rats through modification of different anxiety parameters in the two groups and increased locomotor activity of rats.
4. Discussion
- According to the data obtained, microinjection of a 5-HT1A agonist (8-OH-DPAT, 0.3 µg/0.5 µL) caused higher percentage of open arm time in LA rats compared to the saline injection. But it is worth to mention that a the percentage of open arm time as well as the percentage of open arm entries decreased in LA rats on Trial II compared Trial I after any treatments. It is widely known that the number of open arm entries and open arm time decreased during the retest in the EPM without any injections [14, 6, 31]. Moreover, in our previous work we have shown that the number of entries and time spent in open arms also decreased in the intact/non-surgerized LA rats, but not in HA animals [35]. We assume that higher percentage of open arm time in LA rats compared to saline on Trial II in EPM may be considered as evidence of anxiolytic effects of the 8-OH-DPAT [7, 39]. At the present work microinjection of 8-OH-DPAT increased the time to leave an open arm in ETM in LA rats, which is considered as evidence of panicolytic effects of the drug [39, 46].Literature provides contradictory data on the effect of the 5-HT1A receptor agonist on animal behavior. Local administration of 1.3, 2.6, 5.3 µg /0.2 µL 8-OH-DPAT into basolateral amygdala has been demonstrated to increase the latency to escape from open arm of elevated T-maze (panicolytic-like effect), decreased the time to leave enclosed arm of elevated T-maze and increased the time spent in the light compartment of light-dark box (anxiolytic effect) [39]. At the same time, administration of 0.1 µg /0.2 µL 8-OH-DPAT did not influence the rat behavior in ETM and light-dark box [39]. In another work, local administration of 8-OH-DPAT (2.6, 5.3 µg /0.2 µL) in the amygdala impaired inhibitory avoidance of the open arms but did not change escape performance from one of the open arms of ETM [47]. It should be noted that in a work by Strauss et al. [39], similar to the current study, animals were placed into an isolated open arm of the ETM for 15–30 min 24 h before the testing, which is considered to increase the sensitivity of behavior in ETM to administration of anxiolytics [46]. Anxiolytic effect of 8-OH-DPAT on the animal behavior was also detected in the Vogel conflict test [39], as well as in the experiment on tonic immobility in guinea pigs [18]. Literature data concerning the effect of 8-OH-DPAT on animal behavior in EPM are even more contradictory. A number of reports evidence the absence of the effect of 8-OH-DPAT (50–200 ng/0.5 µL or 0.6, 1.3, 2.6 µg/0.2 µL) on the behavior in EPM upon local injection into the amygdala [12, 45]. In other works, local injection of 8-OH-DPAT (1.8 µg/0.4 µL) in the basolateral amygdala caused anxiogenic effect on the behavior in EPM by decreasing the percent of open arm entries and open arm time [28]. Also, anxiogeniс effect of 8-OH-DPAT was demonstrated in a social interaction test in rats [12]. Therefore, according to the literature data, the effect of 8-OH-DPAT on the animal behavior could depend on the dose applied, as well as on the conditions of the experiment [13, 14, 46]. Besides, there are data that the effect of administration of serotoninergic agents may depend on the experimental model of anxiety used [2, 11, 12]. Different behavioral tests are assumed to model different types of anxiety that are regulated by different brain structures or different receptors involved. Anxiolytic effect of 8-OH-DPAT revealed by our work can be explained by activation of 5-HT1A heteroreceptors, which led to decrease in neuronal activity in basolateral amygdala [13, 38]. Apparently, the activating effects of amygdala on periaqueductal grey matter weakened also, which led to decrease in panic-like behavior.In our work, injection of an antagonist in the amygdala influenced both rat groups affecting different behavioral parameters. Intra-amygdala injection of WAY-100635 (0.2 µg/0.5 µL) decreased the percentage of open arm entries and increased the latency to first enter an open arm in HA rats, suggestive of an anxiogenic effect [34]. In LA rats, injection of the antagonist in the amygdala increased the number of looking out of the enclosed arms and stretched attend postures (risk assessment behavior), as well as the number of enclosed arm entries in the EPM, which is interpreted as an evidence of enhancement in anxiety and locomotor activity respectively [14, 34]. It is believed that risk assessment behavior (looking out of enclosed arms, stretched attend postures and etc.) is closely related to fear/anxiety and may even be more sensitive to anxiety modulating drugs than avoidance related measures (open arm entries and open arm time) [6, 34]. As it has been demonstrated previously, administration of WAY-100635 in the amygdala or hypothalamus did not influence the rat behavior in ETM and light-dark box but blocked anxiolytic and panicolytic effects of 8-OH-DPAT [25, 39]. However, there are data that systemic administration of WAY-100635 increased the latency to leave enclosed arm of ETM, suggestive of an anxiogenic effect [43]. The discrepancy between the literature data and our results can be explained by the fact that the effect of the agents on behavior can depend to a great extent on the experimental conditions (light level, time of the experiment, etc.), as well as the design of the maze [14]. Moreover, according to our data the effect of the 5-НТ1А receptor antagonist could depend on the individual features of the animals' behavior and be manifested selectively in rats with either high or low anxiety level, and not in all population analyzed. This phenomenon could also be the reason for the above-mentioned discrepancy.One may assume that anxiogenic effect of WAY-100635 is associated with the fact that when 5-НТ1А receptors in the amygdala are blocked, other receptor types start to play the leading role. Thus, serotoninergic transmission in amygdala has been shown to be performed not only through the 5-НТ1А receptors, but 5-HT2A/2C and 5-HT3 receptors as well [38]. In studies with extracellular registration of neuronal activity in amygdala, agonists of the 5-HT2 and 5-HT3 receptors have been shown to increase the firing rate of amygdala neurons while 5-HT1A slowed it down [38]. Local injection into amygdala or systemic administration of 5-HT2 and 5-HT3 receptor agonists produced anxiogenic effects in EPM, ETM, and in open field [1, 5, 8, 44]. The balance of inhibitory 5-HT1A and excitatory 5-HT2A receptors has been demonstrated to play an important role in regulation of glutamatergic and GABAergic neuron activity in amygdala, hippocampus, and frontal cortex [23].In the current study, we found that HA and LA rats are characterized by different susceptibility to intra-amygdala injection of 5-HT1A receptors agonist and antagonist. Administration of 8-OH-DPAT caused anxiolytic and panicolytic effects only in LA rats without affecting the behavior of HA animals. Injection of WAY-100635 caused anxiogenic effect in both groups of animals, however the effect differed in LA and HA rats. There are few works on the specific features of serotoninergic ligand effects on animals with different level of anxiety and fear in the literature. In the work with systemic administration it was shown that rats with different duration of avoidance of the open arms in T-maze rats differ in their sensitivity to serotonergic drugs [43]. The 5-HT1A agonist 8-OH-DPAT decreased the duration of avoidance behavior in rats characterized by initially larger duration of avoidance behavior, but increased the duration of this behavior in rats with lower duration of avoidance behavior. It is interesting to note that the 5-HT1A antagonist WAY100635 affected the behavior of both types of rat. However, the onset of the effects of WAY-100635 was faster in rats with larger duration of avoidance behavior, than in rats with lower duration of avoidance behavior. In our work WAY-100635 also affected the rats of both groups. However, WAY-100635 produced more significant anxiogenic effects on the behavior of HA than in LA rats. It was found that rats with low unconditioned startle response amplitude showed more susceptible to activation of 5-HT1A receptor in the basolateral amygdala or periaqueductal gray matter and animals with high startle amplitude, to 5-HT1A receptor activation in the prelimbic cortex [10]. The authors suggested that in rats with different startle response the role of 5-HT1A receptors in various brain structures is different.One of the reasons of different sensitivity of HA and LA rats to the effect of the 5-HT1A receptor ligands can be the differences in the density of the receptors in various rat groups; most probably, in LA rats it should be higher than in HA animals. For example, decrease in expression of 5-HT1A receptors in amygdala of mice has been demonstrated to result in decrease in the time spent in the open arms of the EPM and in the center of an open field [21]. Positron-emission tomography was used to show that binding potential of selective 5-HT1A receptor radioligand ([carbonyl-11C]WAY) is lower in amygdala, some areas of cortex, and raphe nucleus in patients suffering from social anxiety disorders [17], and negatively correlates with the level of anxiety in healthy patients [40]. Yet, to confirm the supposition on differential density of 5-HT1A receptors and their binding potential in HA and LA rats, further studies using immunohistochemistry and radioligand-based methods are required.
5. Conclusions
- Activation of 5-HT1A receptors in basolateral amygdala through local administration of an agonist (8-OH-DPAT, 0.3 µg/0.5 µL) influenced only the behavior of LA rats (anxiolytic effect in EPM and panicolytic effect in ETM). Behavior of HA animals did not change after injection of the agonist.Inhibition of 5-HT1A receptors in basolateral amygdala through local administration of an antagonist (WAY-100635, 0.2 µg/0.5 µL) caused anxiogenic effect on the behavior of HA and LA rats in EPM, however, the agent affected different behavioral parameters in the two groups of animals. In HA rats, along with the anxiogenic effect, enhancement of locomotor activity was observed.LA rats were more sensitive to activation of 5-HT1A receptors of the amygdala than the HA ones.
ACKNOWLEDGEMENTS
- We would like to thank Dr. Natalia R. Kuznetsova (Institute of Bioorganic Chemistry of the Russian Academy of Sciences) for assisting in preparation of this manuscript. This work was supported by the Russian Science Foundation grant 14-25-00072.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML