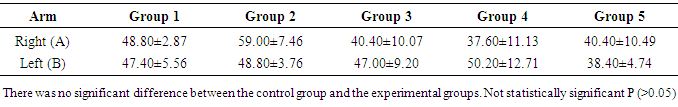-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2017; 6(1): 1-4
doi:10.5923/j.neuroscience.20170601.01

Neurobehavioural Effects of Some Artemisinin-Combination Therapies in Prophylactic Murine Malaria Model
Koofreh G. Davies1, Innocent A. Edagha2
1Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Nigeria
2Department of Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Nigeria
Correspondence to: Koofreh G. Davies, Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Despite the widespread use of Artemisinin combination therapies in the treatment of malaria, there is no available study on the effect of these anti-malarials on neurobehaviour. This study was therefore designed to investigate effects ACTs on anxiety T-Maze and cognition. Twenty five female albino mice weighing 20-26 g were used for this study. Animals were acclimatized and randomly selected into five groups. Light and Dark Box was used to test for anxiety while cognition was tested for with T-maze. Result showed no derangement in any of the neurobehavioural parameters tested for. This result indicates that malaria infection may not affect anxiety and cognition in subjects who currently on prophylactic ACTs. Also, Dihydroartemisinin/Piperaquine, Artemether/Lumefantrine and Artesunate/Amodiaquine did not affect cognition and anxiety at clinical dosage.
Keywords: Artemisinin-Combination Therapy, Prophylaxis, Plasmodium berghei, Anxiety, Cognition
Cite this paper: Koofreh G. Davies, Innocent A. Edagha, Neurobehavioural Effects of Some Artemisinin-Combination Therapies in Prophylactic Murine Malaria Model, Research in Neuroscience , Vol. 6 No. 1, 2017, pp. 1-4. doi: 10.5923/j.neuroscience.20170601.01.
Article Outline
1. Background of the Study
- Malaria is a mosquito-borne infectious disease affecting humans and other animals caused by parasitic protozoa belonging to the Plasmodium type. The mosquito bite introduces the parasite from the mosquito's saliva into a person’s blood (WHO, 2014). The symptoms of malaria typically include fever, fatigue, vomiting, and headaches. In severe cases it can cause yellow skin, seizures, coma, or death. Symptoms usually begin ten to fifteen days after being bitten. If not properly treated, people may have recurrences of the disease month’s later (Beare et al.., 2006). Five species of Plasmodium can infect and be spread by humans. Most deaths are caused by P. falciparum, because P. vivax, P. ovale, and P. malariae generally cause a milder form of malaria. The specie P. knowlesi rarely causes disease in humans (Caraballo, 2014; WHO, 2014). Malaria is typically diagnosed by the microscopic examination of blood using blood films (Sutherland and Hallent, 2009). The recommended treatment for malaria is a combination of antimalarial medications that includes an artemisinin. The second medication may be mefloquine, lumefantrine, or sulfadoxine/pyrimethamine. Quinine along with doxycycline may be used if an artemisinin is not available (WHO, 2010).Artemisinin and its semi-synthetic derivatives are a group of drugs that possess the most rapid action of all current drugs against Plasmodium falciparum malaria (WHO, 2006). Artemisinins can be used alone, but this leads to a high rate of recrudescence (return of parasites) and other drugs are required to clear the body of all parasites and prevent recurrence. The WHO has recommended that artemisinin combination therapies (ACT) should be the first-line therapy for P. falciparum malaria worldwide (Douglas et al., 2010; WHO, 2006).There were a lot of concerns regarding the use of artemisinins following reports that administration of high and prolonged doses of artemisinins in laboratory animals cause neurotoxicity (Nontprasert 2002). However, many other studies have also reported that the artemisinins are very safe when administered at clinical dose regimen (Meshnick, 2002). In some earlier studies, we also demonstrated that artesunate and artemether at clinical doses did not neurobehavioral parameters in laboratory animals (Davies et al., 2012; Davies and Akpan 2016). Generally, there is lack of data on neurological or neurobehavioral effects of artemisinin combination drugs. Studies examining neurobehavioral effects of anti-malarials are not common, as most studies tend to concentrate on the effects of these drugs on malaria parasite alone. Moreover, the few reports available are studies done on healthy animals. Our study appears to be the first to examine the effects of ACTs on malaria infected mice. The aim of this study is to compare the cognitive and anxiety effects of ACTs in prophylactic malaria mice model.
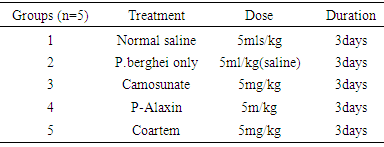
2. Materials and Methods
- Care for AnimalsTwenty five female albino mice weighing 20-26 g were housed in groups of five in cages under standard laboratory conditions and fed with pellet feed. Good quality water was given and the beddings were changed regularly.Preparation of DrugsArtemisinin based combination therapy namely; Dihydroartemisinin/Piperaquine Artemether/Lumefantrine and Artesunate/Amodequine were purchased from a reputable Pharmacy in Uyo, Akwa Ibom State, Nigeria. The drugs were prepared as stated below (1) 20mg tablet of Arthemeter/Lumefantrine was dissolved in 50ml of distilled water to produce a stock solution of 0.4mg/ml.(2) 40mg tablet of Artesunate/Amodiaquine was dissolved in 40ml of distilled water to produce a stock solution of 1mg/ml.(3) 100mg tablet of Dihydroartemisinin/Piperaquine was dissolved in 100ml of distilled water to produce a stock solution of 1mg/ml.Acquisition of Donor MiceParasite Plasmodium berghei was obtained from the animal house of Basic Medical Science University of Uyo, Uyo. A standard inoculum of 1x107 of parasitized erythrocytes from a donor mouse in volumes of 0.4ml was used to infect the experimental animal intraperitoneally.
|
3. Results
|
|
4. Discussion
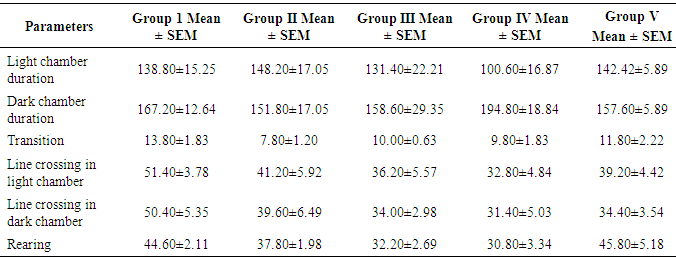
- This is about the first study to examine the effects of ACTs on mice infected with malaria parasite. Specifically, the study evaluated effects of the ACTs on cognition and anxiety. As stated above the ACTs are the current dugs recommended by the WHO for the treatment of both complicated and uncomplicated malaria. ACTs thus deserve more scrutiny because of their widespread usage. T-maze was used to evaluate spatial working memory while anxiety was assessed with light dark transition box. As shown in the result, the time spent in the reference arm of the T-maze was not significantly different between the control group and the experimental groups. The absence of memory deficit in the treated groups suggests that the ACTs used in this study namely, Dihydroartemisinin/Piperaquine Artemether/ Lumefantrine and Artesunate/Amodiaquine, did have any untoward effect on short-term memory. Our earlier studies, using Morris water maze, showed that daily, oral administration of 60mg/kgbwt of artemether for 28 days did not cause memory impairment (Davies et al., 2012). Similar observation was seen in artesunate at same dose level and duration (Davies and Akpan, 2016). Our finding, however, is not in support of an earlier report which showed that Artesunate/Amodiaquine caused impaired short-term memory (Onalapoolakunle et al., 2013). The reason for the difference between our report and the latter is not quite obvious. The result also showed no memory impairment in the untreated mice. This finding is not surprising because on day 7 that the memory test was performed, the untreated mice did not show signs of cerebral malaria, as documented in the SHIRPA protocol (Rodgers, et al., 2001). However, what is not clear, is the reason the animals did not develop cerebral malaria by this time contrary to some reports that showed that cerebral malaria usually occur from 5 days post infection. In another study of ours (under review), Plasmodium beighei berghei infected albino mice showed signs of cerebral malaria by day 9 while mortality was noticed on day 11 but Desruisseaux et al. 2008 demonstrated impaired short term memory 7 days and 75% mortality by day 11 after infection in Plasmodium beighei ANKA infected C57BL/6 mice. The above authors used novel object recognition and neural systems underlying episodic memory to establish their findings. The reason for the difference between our study and the one cited above may be due to the fact that we worked on a different strain of mice. While the study employed C57BL/6, we used albino mice and there may be strain differences. Secondly, our study made use of Plasmodium beighei beighei whereas the study employed Plasmodium beighei ANKA. The latter strain is reported to be more virulent than the former. Finally, reduced virulence may result from continuous cycle of passage, of the parasite. In this study the light and dark box was used to evaluate anxiety and fear. For the purpose of this research the parameters considered were the time duration spent in the light and dark chamber of the box, rearing, number of transition and line crossing. Except for rearing, there was no significant difference between the control and the experimental groups. The light/dark test is based on the innate aversion of rodents to brightly illuminated areas and on the spontaneous exploratory behaviour of rodents in response to novel environment and light (Crawley and Goodwin, 1980; Lister, 1987; Bourin and Hascoêt, 2003). The exploratory activity reflects the combined result of these tendencies in novel situations. Thus, in increase in behaviours in the white part of the light/dark test, indicates decrease anxiety. An increase in transitions without an increase in spontaneous locomotion is considered to reflect anxiolytic activity. The lack of significant difference in these parameters, between the control and the experimental groups, indicates that neither malaria infection nor ACTs affected anxiety in this study. Our observations appear to support report by Desruisseaux et al. 2008, which showed that malaria did not affect exploration of object and environment. Exploration not being affected is an indication that anxiety was not affected since change in anxiety status is usually accompanied change in exploration. As mentioned above rearing was the only parameter that was affected in the light/dark box test. Rearing was significantly lower in the groups that received artesunate/amodiaquine and dihydroartemisin/piperacin combinations. Decrease rearing denotes decrease exploration and increase anxiety. This observation, taken alone would tend to suggest that these drugs caused anxiety and thus reduce exploration. However, this finding alone is not sufficient to arrive at such conclusion. More so, a study by Onalapoolakunle et al. (2013) on the contrary, showed that artesunate/amodiaquine caused reduced anxiety and fear.
5. Conclusions
- Findings in this study showed that indicates that malaria infection may not affect anxiety and cognition in subjects who currently on prophylactic ACTs. Also Arthemeter/Lumefantrine, Artesunate/Amodiaquine, and Dihydroartemisinin/Piperaquine cause did not affect anxiety and cognition at clinical doses.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML