-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2016; 5(1): 15-23
doi:10.5923/j.neuroscience.20160501.03

The Effects of Exercise on Cognitive Function, Balance, and Salivary Brain Derived Neurotrophic Factor in Healthy Individuals – A Pilot Study
Joshua McGeown1, Paolo Sanzo1, Carlos Zerpa1, Simon Lees2, Sarah Niccoli2
1School of Kinesiology, Lakehead University, Thunder Bay, Canada
2Northern Ontario School of Medicine, Lakehead University, Thunder Bay, Canada
Correspondence to: Paolo Sanzo, School of Kinesiology, Lakehead University, Thunder Bay, Canada.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

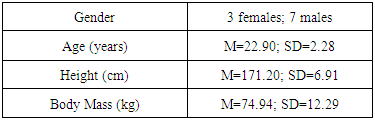
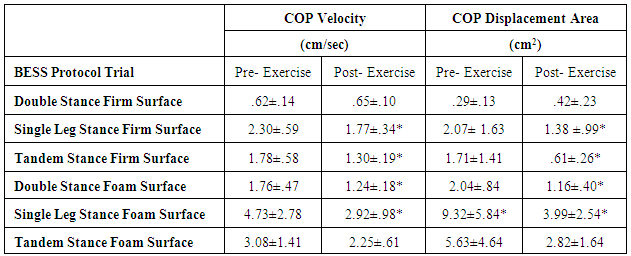
Objective: The purpose of this pilot study was to explore the effect of a four week exercise program on physiological, cognitive, and balance variables and salivary brain-derived neurotrophic factor (BDNF) concentrations in a sample of healthy, physically active individuals. Subjects: Ten healthy participants (3 females, 7 males; age M=22.9 years; SD=2.28; height M=171.20 cm; SD=6.91; and body mass M=74.94 kg; SD=12.29) were included. Methods: Subjects completed two assessments (pre- and post-exercise program) and 12 supervised exercise sessions over the course of four weeks (3 sessions/week). The pre- and post-exercise program assessments included the collection of a saliva sample to measure salivary BDNF concentrations followed by anthropometric measures, resting heart rate, and blood pressure; the administration of the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) battery to measure neurocognitive function; and finally the completion of the Balance Error Scoring System (BESS) protocol to measure balance variables including the number of errors, displacement in the centre of pressure (COP), average velocity of COP, and area of COP. The exercise sessions consisted of a warm-up, followed by 20-35 minutes of aerobic activity, and concluding with three trials of static balance exercises. The intensity and difficulty of the aerobic and balance exercises were progressed using pre-determined parameters and progressions over the course of the four weeks. The data were analysed using descriptive statistics and Paired Sample t-Tests. The rejection criteria were set at an alpha level p < .05. Results: Statistically significant changes in reaction time (t(9)=-2.472, p=.035); BDNF concentrations (t(9)=-1.809, p=.05); average velocity of COP (t(9)=4.69, p=.001) and area of COP (t(9)=4.47, p=.002) in double stance on a foam surface; average velocity of COP (t(9)=3.09, p=.01) and area of COP (t(9)=2.28, p=.04) in single leg stance on a firm surface; average velocity of COP (t(9)=2.65, p=.03) and area of COP (t(9)=3.00, p=.015) in single leg stance on a foam surface; and average velocity of COP (t(9)=2.36, p=.04) and area of COP (t(9)=2.49, p=.04) in tandem stance on a firm surface during the BESS protocol were found. No changes in heart rate, blood pressure, memory, or visual motor speed were observed. No significant changes were seen for the BESS protocol during double stance on a firm surface or tandem stance on foam. Conclusions: The findings of the current pilot study revealed that a supervised four week aerobic and balance exercise program administered to a sample of healthy, physically active individuals resulted in improvements in salivary BDNF concentrations, static balance, average velocity, and area of COP measures. This supervised program requires further investigation into the implementation in a concussed or neurologically impaired population to see if similar benefits are evident in cognitive and balance variables, or BDNF concentration levels.
Keywords: Aerobic, Balance, Exercise, Neurocognitive function, Concussion, Salivary brain-derived neurotrophic factor
Cite this paper: Joshua McGeown, Paolo Sanzo, Carlos Zerpa, Simon Lees, Sarah Niccoli, The Effects of Exercise on Cognitive Function, Balance, and Salivary Brain Derived Neurotrophic Factor in Healthy Individuals – A Pilot Study, Research in Neuroscience , Vol. 5 No. 1, 2016, pp. 15-23. doi: 10.5923/j.neuroscience.20160501.03.
Article Outline
1. Introduction
- Concussion is an injury defined as a complex pathophysiological process affecting the brain induced by forces causing linear, rotational, and angular movements of the brain, or a combination thereof [32]. In 2013 the Canadian Community Health Survey reported that 94,000 concussions affected Canadians (58,000 males and 36,000 females) aged 12 years and older between 2009 and 2010 [43]. Symptoms of concussion are highly variable, and no two concussions are alike, even if sustained by the same individual [32]. Due to the variable nature of concussion, symptoms are generally stratified into one of four categories: 1) somatic (physical symptoms); 2) cognitive (thinking and processing symptoms); 3) affective (emotional symptoms); or 4) sleep disturbances [16]. Most individuals who sustain a concussion recover in less than two weeks but 10-20% experience lingering symptoms of concussion beyond two weeks. These enduring and persistent symptoms are diagnosed and labelled as post-concussion syndrome (PCS). Neuropsychological (NP) testing has been stated to be the cornerstone of concussion assessment and management [7, 32]. These NP tests allow researchers and clinicians to objectively and quantitatively evaluate deficits of cognitive function following concussion. Computerized NP batteries have been developed in order to reduce cost and ease the process of assessment and administration. These computerized formats take 20-30 minutes to complete, and several can be administered at once if multiple computers are available [26]. In the case of baseline testing for many patients, sports teams, and athletes, the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) battery appears to be one of the most commonly used computerized NP testing programs used in the case of baseline testing; however, it is not as commonly used in the presence of chronic symptoms due to PCS. It is unclear if computerized NP test batteries such as the ImPACT demonstrate the necessary sensitivity to detect deficits that may be more subtle in PCS than acute concussion. Currently, there is also a lack of a gold standard and agreement on the definition of PCS [4, 25]. This presents a significant challenge for researchers and clinicians to assess, monitor, and rehabilitate PCS, as the condition is not consistently operationalized within the literature. Furthermore, little is known about the underlying mechanisms that result in persistent symptoms progressing from an acute concussion to PCS.It is hypothesized that the initial concussive injuries may interrupt the delicate synchronization of certain physiological pathways, which fail to resynchronize and result in persistent symptoms. As a result, impaired cognitive function and the ability to maintain balance may last for weeks or months following concussion if the function or sensory integration of the visual and/or vestibular systems is disrupted [14, 31]. Also, damaged white matter cells within the brain have been reported in PCS patients with greater damage being associated with more severe symptoms [9]. Lastly, following concussion, impaired autonomic function has been documented in the form of abnormal heart rate variability and cerebral blood flow [11, 19, 42, 46]. These underlying impairments are especially worrisome when present in young developing neurological systems as these impairments may disrupt the typical developmental processes and result in atypical development with possible resultant long term ramifications on the individual [42]. The vast majority of concussed patients often benefit from cognitive and physical rest during the acute (initial 7-10 day) phase of the concussion [34]. Generally, patients are instructed to engage in cognitive and physical rest which includes no school or work, driving, screen time, chores, physical exercise, or activity that results in perspiration [34]. This period of rest is hypothesized to allow the body to divert necessary energy stores to resolving the metabolic disturbances documented to occur following the neurometabolic cascade of concussion [12, 42]. Exercise during this acute phase following concussion has been observed to exacerbate the symptoms of concussion [11, 32]. There is, however, no specific method of progressing rehabilitation in PCS patients following the acute period and rest is generally continued as the prescribed treatment and standard of care [34, 37, 38, 47]. Typically, rest is often prescribed as the treatment of choice because it is conservative and can be used in combination with education, reassurance of positive expectations of recovery, and suggested coping strategies [5]. However, there is limited evidence that extended rest, beyond 7-10 days, will benefit those with PCS positively. An extended period of cognitive and physical rest beyond the first 7-10 days may result in improvement in some cases or, conversely, increased physical, psychological, and/or social stress in the form of physical deconditioning, hyperawareness of the symptoms, or loss of productivity at work or school [21, 33, 36].Early evidence now suggests the benefits of an active exercise program for those experiencing PCS due to the growing link within the literature regarding exercise and neuroplasticity and the ability of neurons to alter the strength and efficacy of pre-existing synapses in addition to forming new synaptic connections [1, 48]. Aerobic exercise has also been reported to increase concentrations of neurotrophic factors and biological markers within the body which aid in the regulation of neuronal survival, development, function, and plasticity [17]. Brain-derived neurotrophic factor (BDNF) is one of the identified neurotrophic factors of interest, and the reported effects specific to BDNF demonstrate its potential as a non-pharmacological assist to benefit impaired neurological function [15]. Exercise has been documented to be one way to increase BDNF levels and has been correlated with improvements in neurological functions such as learning and memory, and in altering an individual’s mood [13, 10, 41]. Increased concentrations of BDNF through the administration of aerobic exercise has also been reported to improve cognitive function and memory in Alzheimer’s Disease, stroke, and animal models of concussion [22, 30, 42].To date, it appears that Leddy et al. [23] were the first and only group to investigate the possible application of aerobic exercise in human subjects as an intervention strategy for PCS. Leddy and colleagues had participants with PCS perform an exercise protocol in order to determine the heart rate intensity in which participant symptoms were exacerbated. Afterwards, an individualized stationary bike aerobic exercise program was developed for each participant tailored to his/her recorded symptom exacerbation threshold. Participants were provided with instructions and exercise intensities below his/her symptom threshold. Brain activation was then measured using functional magnetic resonance imaging (fMRI) before and after the exercise program. At the baseline assessment, participants in the biking group were no different in terms of brain activation patterns than a control group of participants with PCS assigned to a stretching only group. The brain activation patterns observed in both PCS groups were significantly different, however, than the activation patterns seen in a group of healthy non-concussed individuals. After the completion of the exercise program, the PCS biking group displayed improved activation patterns that were the same as the healthy individuals, whereas the PCS stretching group remained significantly different. Furthermore, participants in the PCS biking group were able to elevate his/her heart rate to their theoretical maximum without experiencing any symptom exacerbation, while the stretching group was unable to do so [23]. These results highlight the need for further investigation into the utility of exercise to rehabilitate patients that have persistent concussion symptoms. Similarly, based on the evidence within other areas of the literature, it appears that BDNF may be a biomarker of interest to monitor change in brain health at the time of baseline or initial assessment or following the implementation of rehabilitation programs. Supervised and controlled exercises may promote improvements to stimulate/regulate mechanisms of neuroplasticity. In turn, this may provide a stimulus to facilitate the internal physiological environment for the brain to heal itself via plasticity. More research, however, is required on the feasibility of implementing the assessment of this biomarker in combination with other clinical evaluative tools and the effect that exercise may have on such variables. Therefore, the purpose of this pilot study was to investigate the effects of a supervised and structured four week aerobic exercise and balance exercise program on resting heart rate, blood pressure, cognitive function, balance, and salivary BDNF concentrations in a sample of normal healthy individuals.
2. Methods
2.1. Subjects
- Ten healthy participants absent of any debilitating injury or condition that would prevent them from exercising were recruited for the pilot study. Participants ranged in age between 20 and 29 years and all participants were regularly physically active for 150 minutes or more on a weekly basis prior to entering the study (see Table 1).
|
2.2. Procedure
- After obtaining institutional ethical approval, prospective participants were recruited using convenience sampling. Informed consent was obtained from prospective participants and afterwards an initial screening assessment was completed in which age, height, and body mass was measured and recorded. Prior to collecting the saliva sample, participants were instructed to refrain from brushing his/her teeth, smoking, and consuming food or drink within two hours of the sample collection and avoiding the consumption of any alcohol 12 hours prior to collection [35, 6, 29]. Samples of salivary BDNF were collected in the morning between 8:00-9:00 a.m. to minimize the effect of any diurnal variation [28, 44, 49]. The pre-treatment data collection began by obtaining a 2mL saliva sample to assess BDNF concentrations. Participants passively drooled into a microcentrifuge tube to provide the sample, which was then put on ice. A modified methodology from Mandel et al. [28] was followed for processing. Briefly, samples were centrifuged in a precooled centrifuge at 4000 rpm for 15 minutes. Supernatant was then aliquoted into tubes to which protease inhibitor cocktail was added (Sigma Aldrich, St. Louis, MO) to a final dilution of 1:100. These saliva samples were then frozen and stored at -80°C.Next, participants completed a neurocognitive assessment using the computerized ImPACT battery. The ImPACT was used to assess verbal memory, visual memory, reaction time, and the visual motor speed of participants. Schatz, Pardini, Lovell, and Podell [39] explored the sensitivity and specificity of the ImPACT battery when classifying concussed and non-concussed individuals and reported 82% sensitivity to correctly classifying concussed individuals, and 89% specificity for ruling out concussion in a healthy control group. Schatz revisited the psychometric properties of the ImPACT in 2010, evaluating the test-retest reliability of the ImPACT composite scores over two years. Schatz [40] reported that the visual motor speed composite demonstrated the best test-retest reliability (intra-class correlation, ICC, = 0.74), followed by the reaction time composite (ICC= 0.68), visual memory (ICC= 0.65), and, lastly, verbal memory (ICC= 0.46). The ImPACT battery has demonstrated good construct validity as well when compared with traditional paper-and-pencil NP tests [26]. Lastly, Allen and Gfeller [2] compared the ImPACT to traditional paper-and-pencil NP tests using a factor analysis approach; the authors reported good overall concurrent validity with five factors explaining 69% of the variance in ImPACT scores.After the ImPACT battery was completed, participants were asked to perform the Balance Error Scoring System (BESS) protocol on an AMTI force platform (see Figure 1).
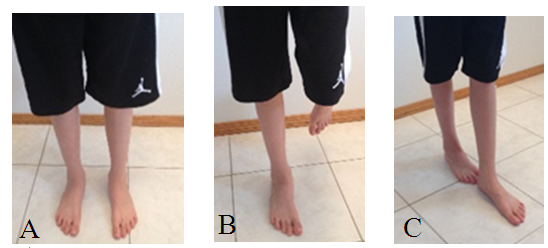 | Figure 1. Double leg stance (A); Single Leg Stance (B); and Tandem Stance (C) |
2.3. Data Analysis
- Descriptive statistics were used to compare the mean and standard deviations for individual ImPACT battery and BESS protocol scores and salivary BDNF values. Data analysis was completed using IBM SPSS 20 to evaluate any change that occurred following the exercise program. Changes observed in the dependent variables were assessed for statistical significance using Paired Samples t-Tests, with an alpha level of .05. The analysis evaluated the effect of the exercise program on the dependent variables (resting heart rate; resting blood pressure; ImPACT verbal memory, visual memory, visual motor speed, and reaction time scores; salivary BDNF concentration; and BESS total score, COP displacement, and average velocity of COP) comparing pre- and post-intervention scores and values. Log transformation correlational analysis was also completed to examining pre- to post-intervention BDNF levels.
3. Results
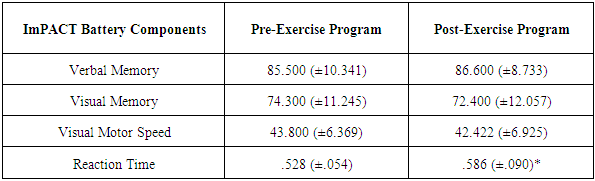
- There were no significant differences in resting heart rate (t(9)=2.08, p=.07), resting systolic blood pressure (t(9)=.67, p=.52), or resting diastolic blood pressure (t(9)=.71, p=.49). Additionally, there were no significant changes in cognitive functions of verbal memory (t(9)=-.27, p=.79), visual memory (t(9)=.60, p =.56), or visual motor speed (t(9)=1.26, p=.24) scores with ImPACT testing. Notably, there was a statistically significant increase in reaction time (t(9)=-2.47, p=.04) after the exercise program while performing cognitive tasks (see Table 2). The mean reaction time for participants before the exercise program was .53 +/- .05 seconds per response, whereas after the program this increased to .59 +/- .09 seconds per response (see Figure 2).Salivary BDNF concentrations were observed to significantly increase in response to the exercise program (t(9)=-1.809, p=.05). Saliva sample mean BDNF concentration levels at baseline pre-intervention were 137.25 +/- 88.07 pg/ml, while the mean concentration post-intervention was 199.04 +/- 80.13 pg/ml (see Figure 3). Also, it should be noted that in participants with lower initial BDNF levels, the largest increase was evident post-intervention (see Figure 4).
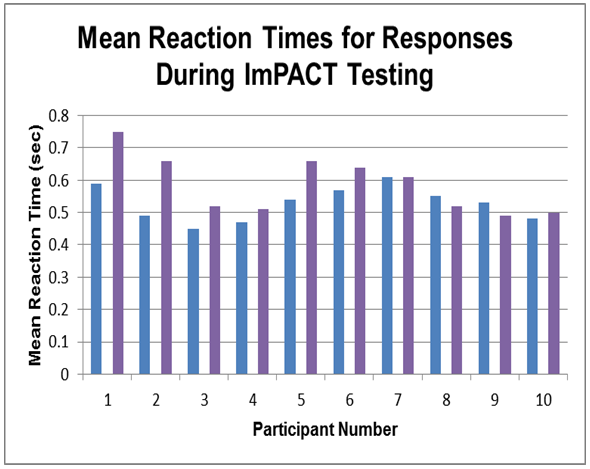 | Figure 2. Changes observed in mean reaction time (sec) during the ImPACT test battery.  – pre-intervention mean reaction times; – pre-intervention mean reaction times;  - post- intervention mean reaction times - post- intervention mean reaction times |
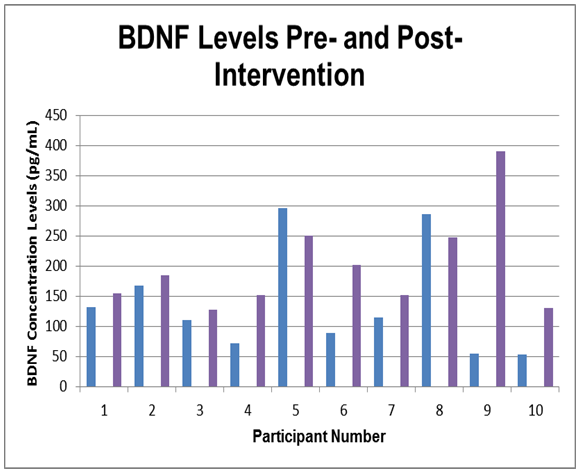 | Figure 3. Changes observed in the BDNF concentration levels (pg/mL).  - BDNF levels pre-intervention; - BDNF levels pre-intervention;  - BDNF levels post-intervention - BDNF levels post-intervention |
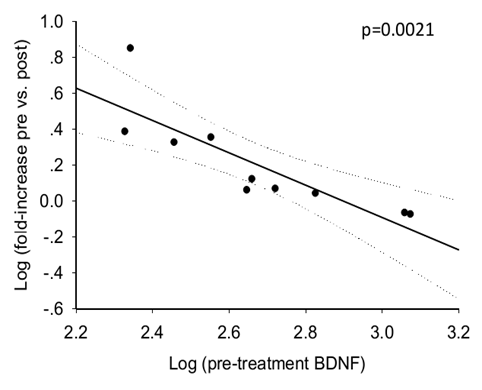 | Figure 4. Log transformation correlation between pre-treatment BDNF concentration and the increase due to the intervension |
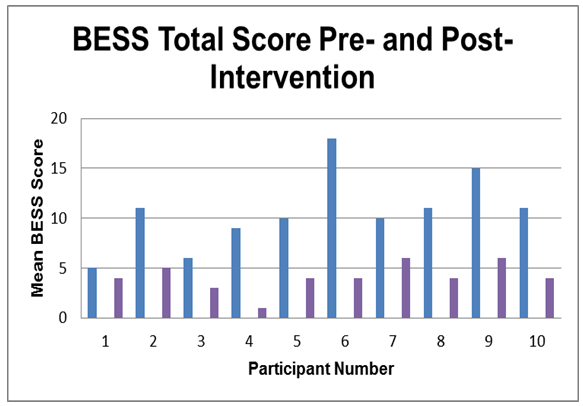 | Figure 5. Changes observed in the BESS protocol total scores.  – pre-intervention BESS score; – pre-intervention BESS score;  - post-intervention BESS score - post-intervention BESS score |
|
4. Discussion
- The purpose of this pilot study was to investigate the effects of a supervised and structured four week aerobic and balance exercise program on resting heart rate, blood pressure, cognitive function, and balance in a healthy sample of individuals as a preliminary study for proof of concept to be later applied to a PCS population. While minimal to modest changes were initially hypothesized to be observed following the exercise program in this population, several distinct results were found. No significant changes in resting heart rate, resting systolic, or diastolic blood pressure were found. This result was not unexpected due to the healthy, physically active sample recruited for the exercise program. Regular physical activity is associated with optimal blood pressure and heart rate [8]. Activity strengthens the heart and vessels, in addition to improving the control of the autonomic nervous system’s regulation of heart rate and blood pressure [8]. If an individual experiencing PCS was prescribed a period of cognitive and physical rest for weeks or months, this may result in physical deconditioning, which may impair the regulation of heart rate and blood pressure. Less than ideal regulation of heart rate and blood pressure has been hypothesized as a reason why individuals with PCS continue to present with symptoms long after the typical recovery period of 7-10 days [11]. Therefore, the prescription of this exercise program may result in different findings in the future application to a patient population in which heart rate and blood pressure are impaired. No significant differences were observed in verbal memory, visual memory, or visual motor speed with ImPACT testing, however, reaction time significantly increased when completing the ImPACT battery. The increased reaction time may be explained by a learning effect and the fact that participants consciously took more time to react to the stimuli in order to avoid incorrect responses during the post-treatment test session. However, it is notable that although reaction time increased, the delay in reaction time was very small (.06 seconds). Even though participants were slower to respond to the tasks, responses and scores were no better than those measured pre-exercise. Concentrations of salivary BDNF were significantly increased in response to the four week exercise program. This was a particularly interesting finding as BDNF has been reported to demonstrate the potential as a non-pharmacological intervention to benefit impaired neurological function [15]. Furthermore, BDNF has been documented as a major factor in the regulation of neuroplasticity; the ability of the brain to undergo changes in strength of mature synaptic connections, as well as the formation of synapses in adult and developing brains [15, 48]. The phenomenon of neuroplasticity also extends to the regeneration of synapses following an injury to the central nervous system, such as is the case with a concussion. Aerobic exercise has been observed to be an effective method of increasing BDNF concentrations [15, 22, 30, 45].The prescription of aerobic exercise to healthy adolescents and college students has been observed to result in improved cognitive function, faster reaction times, and verbal learning [24, 45]. These findings were not replicated within the current study; however, this may be attributed to the fact that the ImPACT battery has been designed to assess cognitive function in concussed individuals and not necessarily normal healthy controls. Therefore, the ImPACT may lack adequate sensitivity to detect more subtle changes in a healthy population.In animal models of Alzheimer’s Disease, voluntary treadmill exercise upregulated BDNF concentrations and was associated with improvements of learning and memory function [22]. Similarly, in animal models of concussion, voluntary treadmill exercise resulted in increased BDNF levels, associated with improved learning, memory, and overall cognitive performance [30]. The findings of the current study support these findings in that aerobic exercise and balance retraining resulted in increased salivary BDNF. It should also be noted that in participants with lower initial BDNF levels, the largest increase was evident post-intervention. However, the timing and administration of exercise may be crucial for the prescribing clinician, as aerobic exercise prescribed within the acute phase of concussion (initial 7-10 days after injury) may result in increased symptoms and possibly decreased BDNF concentrations. Conversely, delayed administration of aerobic exercise following the acute phase resulted in upregulation of BDNF associated with improved cognitive function in mice [42]. Leddy et al. [23] appeared to be the only group to date to administer aerobic exercise to a sample of human participants with PCS. While the authors did not measure BDNF concentration levels within their study, they did report that the prescription of aerobic exercise improved cognitive impairments associated with PCS. Prior to the completion of the exercise program, participants with PCS were observed to have significantly different patterns of brain activation during cognitive tasks, as measured by fMRI when compared to healthy controls. Following the aerobic exercise program, participants with PCS improved, exhibiting similar brain activation patterns during cognitive tasks as the healthy controls [23]. The structure and administration of the exercise protocol within the current study elicited significant increases in salivary BDNF, despite all of the participants being healthy and regularly physically active individuals. Therefore, it seems plausible that in a sample of individuals with PCS a similar exercise program may increase BDNF concentrations, facilitate neuroplasticity, and by extension, lead to PCS symptom reduction. The findings of the present study warrant further investigation on the effect of aerobic exercise on BDNF concentration and cognitive function in individuals with PCS. Significant reductions in the BESS total scores were also observed in the current study. The BESS protocol is a widely used clinical tool for the assessment of static balance following concussion, with established normative data collected from healthy, post-secondary aged individuals [18]. Significant reductions in the average velocity of the COP and the area of COP were also observed in four of the six BESS trials. These findings in a healthy population provide evidence that further exploration of a balance retraining program similar to the one administered in the current study may be applied in a subsequent study and possibly aid in improving balance deficits in participants with PCS. Since the exercise program elicited improvements in individuals with no underlying conditions of impaired balance, it seems plausible that similar or greater improvements may be observed in a sample of patients with PCS. Balance deficits associated with PCS are hypothesized to be the result of diminished sensory integration due to the initial concussive injury [3]. Therefore, a progressive exercise program may foster improvements in impaired integration of sensory information by challenging the neuromuscular system to adapt to new demands and, by extension, result in greater ability to maintain balance. The limitations of the current study include the fact that the ImPACT battery was used to measure cognitive functions of healthy participants. The ImPACT is designed to detect deficits in cognitive function in concussed individuals with notable impairments. It is possible that subtle changes in cognitive function may have occurred, which the ImPACT lacked the requisite sensitivity to detect. Second, due to a small sample size, the statistical power of the results from this study was low. Replication of this aerobic and balance exercise program with a larger sample size and control group may reveal different trends within the data undetected within the present study. Lastly, this pilot study only examined healthy individuals who were regularly physically active prior to entering the study. Different results may be found if replicated with a sample of PCS or sedentary individuals. Future studies should investigate the impact of a similar, supervised aerobic exercise and balance retraining program for individuals with PCS, as long as exercise is administered below symptom threshold. If exercise is a possible viable option for PCS rehabilitation, more research regarding the ideal frequency, intensity, time, and type of exercise will have to be investigated. In addition, further investigation into the role BDNF plays as a biomarker in the severity of PCS symptoms or if increased in this biomarker are indicative of improved rehabilitation outcomes, as well as it role in other neurological impairment is warranted. Furthermore, exercise has been reported to act as a potent serotonergic mediator promoting recovery from depression [45]. If exercise and BDNF display positive relationships with improving outcomes and certain symptoms then future studies exploring the effect of exercise on BDNF concentrations in individuals with depression or with other neurological impairments is warranted.
5. Conclusions
- The current pilot study supported the feasibility of a supervised four week aerobic and balance exercise program and demonstrated that the administration of such a program to a sample of healthy, physically active individuals resulted in improvements in salivary BDNF concentrations, static balance, average velocity, and area of COP measures. Further research is required to explore the application of supervised exercise programs in the concussion population or individuals with other neurological impairments.
References
| [1] | Adachi, N., Numakawa, T., Richards, M., Nakajima, S., & Kunugi, H. (2014). New insight in expression, transport and secretion of brain-derived neurotrophic factor: Implications in brain-related diseases. World Journal of Biological Chemistry, 5(4), 409-428. |
| [2] | Allen, B.J., & Gfeller, J.D. (2011). The Immediate Post-Concussion Assessment and Cognitive Testing battery and traditional neuropsychological measures: A construct and concurrent validity study. Brain Injury, 25 (2), 179-191. |
| [3] | Alsalaheen, B., Whitney, S., Mucha, A., Morris, L., Furman, J., & Sparto, P. (2013). Exercise prescription patterns in patients treated with vestibular rehabilitation after concussion. Physiotherapy Research International, 18(2), 100-8. doi:10.1002/pri.1532. |
| [4] | Baker, J. G., Freitas, M. S., Leddy, J. J., Kozlowski, K. F., Willer, B. S. (2012). Return to full Functioning after graded exercise assessment and progressive exercise treatment of postconcussion syndrome. Rehabilitation Research and Practice, 1-7. |
| [5] | Belanger, H. G., Barwick, F. H., Kip, K. E., Kretzmer, T., & Vanderploeg, R. D. (2013). Postconcussive symptoms complaints and potentially malleable positive predictors. The Clinical Neuropsychologist, 27 (3), 343-355. |
| [6] | Ben-Soussan, T. D., Piervincenzi, C., Venditti, S., Verdone, L., Caserta, M., & Carducii, F. (2014). Increased cerebellar volume and BDNF level following quadrato motor training. Synapse, 1-6. |
| [7] | Boake, C., McCauly, S. R., Levin, H.S., Pedroza, C., Constant, C.F., Song, J.X.,… Diaz-Marchan, P.J. (2005). Diagnostic criteria for postconcussional syndrome after mild to moderate traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences, 17(3), 350-356. |
| [8] | Canadian Society for Exercise Physiology. (2013). CSEP-PATH: Physical activity training for health. Ottawa, ON: Canadian Society for Exercise Physiology. |
| [9] | Dean, P., Sato, J.R., Vieira, G., McNamara, A., & Sterr, A. (2015). Long-term structural changes after mTBI and their relation to post-concussion symptoms. Brain Injury, 29 (10), 1211-1218. |
| [10] | Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo A., Chaddock, L.,… Kramer, A. F. (2011). Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA, 108 (7), 3017-22. doi: 10.1073/pnas.1015950108. |
| [11] | Gall, B., Parkhouse, W., & Goodman, D. (2004). Heart rate variability of recently concussed athletes at rest and exercise. Med Sci Sports Exerc, 36(8), 1269-74. |
| [12] | Giza, C. C., & Hovda, D. A. (2001). The neurometabolic cascase of concussion. Journal of Athletic Training, 36 (3), 228-235. |
| [13] | Griffin, E.W., Mullally, S., Foley, C., Warmington, S. A., O’Mara, S. M., & Kelly, A. M. (2011). Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiology & Behaviour, 104 (5), 934-931. |
| [14] | Guskiewicz, K.M. (2001). Postural stability assessment following concussion: One piece of the puzzle. Clinical Journal of Sports Medicine, 11, 182-189. |
| [15] | Hennigan, A., O’Callaghan, R. M., & Kelly, A.M. (2007). Neurotrophins and their receptors: Roles in plasticity, neurodegeneration and neuroprotection. Biochemical Society Transactions, 35 (2), 424-427. |
| [16] | Herring, S. A., Cantu, R. C., Guskiewicz, K. M., Putukian, M., & Kibler, W. B. (2011). Concussion (mild traumatic brain injury) and the team physician: A consensus statement – 2011 update. Official Journal of the American College of Sports Medicine, 43 (12), 2412-22. |
| [17] | Huang, E. J., & Reichardt, L. F. (2001). Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci, 24, 677-736. |
| [18] | Iverson, G. L., & Koehle, M. S. (2013). Normative data for the modified balance error scoring system in adults. Brain Injury, 27 (5), 196-599. |
| [19] | Junger, E. C., Newell, D. W., Grant, G. A., Avellino, A. M., Ghatan, S., Douville, C. M.,… Winn, H. R. (1997). Cerebral autoregulation following minor head injury. J Neurosurg, 86, 425-432. |
| [20] | Karvonen Method. (2007). In the Oxford Dictionary of Sports Science & Medicine 3rd Edition online. Retrieved from http://www.oxfordreference.com/view/10.1093/acref/978019856 8506.001.0001/acref-9780198568506-e-3771. |
| [21] | Kleffelgaard, I., Roe, C., Soberg, H. L., & Bergland, A. (2012). Associations among selfreported balance problems, post-concussion symptoms and performance-based tests: A longitudinal follow-up study. Disability & Rehabilitation, 34(9), 788-794. |
| [22] | Koo, J. H., Kwon, I. S., Kang, E. B., Lee, C. K., Lee, N. H., Kwon, M. G.,… Cho, J.Y. (2013). Neuroprotective effects of treadmill exercise on BDNF and PI3-K/Akt signaling pathway in the cortex of transgenic mice model of Alzheimer’s disease. J Exerc Nutr Biochem, 17 (4), 151-160. |
| [23] | Leddy, J.J., Cox, J.L., Baker, J.G., Wack, D.S., Pendergast, D.R.,… Willer, B. (2013). Exercise treatment of postconcussion syndrome: A pilot study of changes in functional magnetic resonance imaging activation, physiology and symptoms. J Head Trauma Rehabil, 28 (4), 241-249. |
| [24] | Lee, T. M. C., Wong, M. L., Lau, B. W. M., Lee, J. C. D., Yau, S. Y., & So, K. F. (2014). Aerobic exercise interacts with neurotrophic factors to predict cognitive functioning in adolescents. Psychoneuroendocrinology, 39, 214-224. |
| [25] | Legome, E. L., & Wu, T. (2014, Oct 16). Postconcussion Syndrome. Medscape. Retrieved 09/01/2015 from http://emedicine.medscape.com /article/828904-overview |
| [26] | Maerlender, A., Flashman, L., Kessler, A., Kumbhani, S., Greenwald, R.,… McAllister, T. (2010). Examination of the construct valdidity of ImPACT computerized test, traditional and experimental neuropsychological measures. The Clinical Neuropsychologist, 24, 1309-1325. |
| [27] | Mandel, A. L., Ozdener, H., & Utermohlen, V. (2011). Brain-derived neurotrophic factor in human saliva: ELISA optimization and biological correlates. Journal of Immunoassay and Immunochemistry, 32 (1), 18-30. |
| [28] | Mandel, A., L., Ozdener, H., & Utermohlen, V. (2011). Brain-derived neurotrophic factor in human saliva: ELISA optimization and biological correlates. J Immunoassay Immunochem, 32(1), 18-30. |
| [29] | Mandel, A., L., Ozdener, H., & Utermohlen, V. (2009). Identification of pro- and mature brain-derived neurotrophic factor in human saliva. Archives of Oral Biology, 54, 689-695. |
| [30] | Mang, C. S., Campbell, K. L., Ross, C. J. D., & Boyd, L. A. (2013). Promoting neuroplasticity for motor rehabilitation after stroke: Considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Physical Therapy, 93 (12), 1707-1716. |
| [31] | McCrea, M., Guskiewicz, K.M., Marshall, S.W., Barr, W., Randolph, C., Cantu, R.C.,… Kelly, J.P. (2003). Acute effects and recovery time following concussion in collegiate football players: the NCAA concussion study. Journal of the American Medical Association, 290, 2556-2563. |
| [32] | McCrory, P., Meeuwisse, W.H., Aubry, M., Cantu, B., Dvorak, J., Echemendia, R.J., …Turner, M. (2013). Consensus statement on concussion in sport: The 4th international conference on concussion in sport held in Zurich, November 2012. Journal of Sports Medicine, 47, 250-258. |
| [33] | Moore, R. D., Hillman, C. H., & Broglio, S. P. (2014). The persistent influence of concussive injuries on cognitive control and neuroelectric function. Journal of Athletic Training, 49 (1), 24-35. |
| [34] | Moser, R., Glatts, C., & Schatz, P. (2012). Efficacy of immediate and delayed cognitive and physical rest for treatment of sports-related concussion. The Journal of Pediatrics, 161(5), 922-6. doi:10.1016/j.jpeds.2012.04.012 |
| [35] | Mougeot, J. L., Hirsch, M. A., Stevens, C. B., & Mougeot, F. K. (2016). Oral biomarkers in exercise‐induced neuroplasticity in Parkinson's disease. Oral diseases. Oral Diseases, 1-9. |
| [36] | Papa, L., Ramia, M.M., Edwards, D., Johnson, B.D., & Slobounov, S.M. (2015). Systematic review of clinical studies examining biomarkers of brain injury in athletes after sports-related concussion. J Neurotrauma, 32 (10), 661-673. |
| [37] | Patterson, Z.R., & Holohan, M.R. (2012). Understanding the neuroinflammatory response following concussion to develop treatment strategies. Frontiers in Cellular Neuroscience, 6 (58), 1-10. |
| [38] | Sayegh, A. A., Sandford, D., & Carson, A. J. (2010). Psychological approaches to treatment of postconcussion syndrome: A systematic review. J Neurol Neurosurg Psychiatry, 81, 1128-1134. |
| [39] | Schatz, P., Pardini, J., Lovell, M.R., & Podell, K. (2006). Sensitivity and specificity of the ImPACT test battery for concussion in athletes. Archives of Neuropsychology, 21 (1), 91-99. |
| [40] | Schatz, P. (2010) Long-term test-retest reliability of baseline cognitive assessments using ImPACT. American Journal of Sports Medicine, 38 (1), 47–53. |
| [41] | Shih, P. C., Yang, Y. R., & Wang, R. Y. (2011). Effects of exercise intensity on spatial memory performance and hippocampal synaptic plasticity in transient brain ischemic rats. PLos One, 8 (10). doi: 10.1371/journal.pone.0078163 |
| [42] | Shrey, D.W., Griesbach, G.S., & Giza, C.C. (2011). The pathophysiology of concussion in youth. Phys Med Rehab Clin N Am, 22 (4), 577-602. |
| [43] | Statistics Canada. (2013). Canadian Community Health Survey 2010. Retrieved fromhttp://www.statcan.gc.ca/pub/82-624-x/2011001/article/app/11506-03-app3-eng.htm |
| [44] | Tirassa, P., Iannitelli, A., Sornelli, F., Cirulli, F., Mazza, M., Calza, A., Alleva, E., Branchi, I., Aloe, L., Bersani, G., & Pacitti, F. (2012). Daily serum and salivary BDNF levels correlate with morning-evening personality type in women and are affected by light therapy. Rivisita di psichiatria, 47(6), 527-534. |
| [45] | van Praag, H. (2009). Exercise and the brain: Something to chew on. Trends in Neurosciences, 32(5), 283-290. |
| [46] | Vavilala, M. S., Lee, L. A., Boddu, K., Visco, E., Newell, D. W., Zimmerman, J. J., & Lam, A. M. (2004). Cerebral autoregulation in pediatric traumatic brain injury. Pediatr Crit Care Med, 5 (3), 257-263. |
| [47] | Waljas, M., Iverson, G.L., Lange, R.T., Hakulinen, U., Dastidar, P., Huhtala, H.,… Ohman, J. (2015). A prospective biopsychosocial study of the persistent post-concussion symptoms following mild traumatic brain injury. Journal of Neurotrauma, 32 (8), 534-547. |
| [48] | Wells, D.G. (2002). Neuroplasticity. Encyclopedia of Aging. Retrieved from Encyclopedia.com:http://www.encyclopedia.com/doc/1G2-3402200284.html |
| [49] | Yeh, F. C., Kao, C. F., & Kuo, P. H. (2015). Explore the Features of Brain-Derived Neurotrophic Factor in Mood Disorders. PloS one, 10(6), 1-20. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML