-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2015; 4(1): 1-9
doi:10.5923/j.neuroscience.20150401.01
Hyperglycemic Effect on Brain Cholinergic Functions, Oxidative Stress and Protein Expression of Brain Derived Neurotropic Factor (Bdnf) on Cognitive Functions in Streptozotocin Induced-Diabetic Rats
O. A. T. Ebuehi , Dibie D. C.
Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria
Correspondence to: O. A. T. Ebuehi , Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Background: Diabetes mellitus (DM) is one of the most severe metabolic disorders in humans characterized by hyperglycemia due to a relative or an absolute lack of insulin or the action of insulin on its target tissue or both. Many neurodegenerative disorders, such as deficits in learning memory and cognition, are associated with diabetes mellitus. Objective: The objective of the present study was to decipher the effect of hyperglycemia on cholinergic functions, oxidative stress and protein expression of brain derived neurotrophic factor (BDNF) in streptozotocin induced- diabetic rats. Methods: Streptozotocin (STZ)-induced diabetic rats were used for the study. Twelve male Sprague-Dawley rats (130-150g) each were divided into 2equal groups with six rats in each group (diabetic and control). The rats were sacrificed and biochemical parameters, such as blood glucose, C-peptide, total protein, acetyl choline levels, acetyl cholinesterase activity and antioxidant status were determined. The BDNF protein expression and learning, memory and cognitive functions in rats were also determined. Results: The results obtained showed that diabetic rats exhibited decreased acetylcholine level and also an alteration (down regulation) of the expressed BDNF of STZ-induced diabetic rats compared with the control. The diabetic rats also exhibited significantly decreased plasma C-peptide and total protein levels, as well as increased oxidative stress. Conclusions: Data of the study indicate that the streptozotocin induced diabetic rats had increased oxidative stress, decreased acetylcholine levels and down regulation of BDNF protein expression, thereby impairing memory, learning, and cognitive functions.
Keywords: Hyperglycemia, Oxidative stress, Acetylcholine, BNDF, Protein expression, Diabetes
Cite this paper: O. A. T. Ebuehi , Dibie D. C. , Hyperglycemic Effect on Brain Cholinergic Functions, Oxidative Stress and Protein Expression of Brain Derived Neurotropic Factor (Bdnf) on Cognitive Functions in Streptozotocin Induced-Diabetic Rats, Research in Neuroscience , Vol. 4 No. 1, 2015, pp. 1-9. doi: 10.5923/j.neuroscience.20150401.01.
Article Outline
1. Introduction
- Diabetes mellitus (DM) is a major global health problem and it is one of the most severe metabolic disorders in humans. It is characterized by hyperglycemia due to a relative or an absolute lack of insulin or the action of insulin on its target tissue or both (Heller and Macdonald, 1996). Recently, neurological consequences of DM in the CNS have increased considerably. The manifestations of these disorders in diabetic patients include; alterations in neurotransmission and cognitive deficit (Biessels et al., 2001). The streptozotocin-induced diabetic rat is still considered as an important model for the pathophysiology of diabetes mellitus.Evidence is accumulating that people with type 2 diabetes are at risk of developing cognitive impairment (Strachan et al, 2003). This probably is as a result of the synergistic interaction between metabolic derangements associated with diabetes and the structural and functional changes that occur within central nervous system. The dramatic rise in the prevalence of diabetes could precipitate an even greater increase in the number of people who could develop dementia and there are now substantial data which suggest that diabetes, and especially type 2 diabetes, is associated with an increased risk of dementia. (Cukierman et al, 2005). The brain derived neurotrophic factor (BDNF), is one of 4 identified neurotrophins, was originally first identified in the brain (Blurton-Jonesa et al, 2009). In the brain, BDNF promotes the optimum communication between neurons by enhancing “plasticity” at the synapse. The adaptive capacity by the brain is reflected in the ability to integrate information and experiences, and through higher cognitive processing, anchor them into learning and memory formation. The synapse is a critical locus in this adaptive capacity. The integration of learning and subsequent memory formation, and the plasticity that enhances that process, is reflected and promoted by the formation of new synapses that extend the network of communication, and strength of the synaptic connection (Yamada et al, 2002).Since the brain is mainly a glucose-dependent organ, it can be damaged by hyperglycemia induced abnormalities (Blurton-Jonesa et al, 2009, Yamada et al, 2002). This is because the disturbances of neuronal glucose transport and metabolism in hyperglycemia can induce the production of increased amount of free radicals in a diabetic condition and subsequently negatively affect the production of BDNF. The BDNF plays an important role in the survival of neurons, their growth (axons and dendrites), and formation and function of synapse (Blurton-Jonesa et al, 2009). Without sufficient BDNF and other neurotrophic factors, neurons die (Yamada et al, 2002). Therefore, this is a contributing factor for learning, memory and cognitive impairments in diabetic situation, since BDNF promotes the optimum communication between neurons by enhancing “plasticity” at the synapse thereby integrating information and experiences, through higher cognitive processing, and anchoring them into learning and memory formation (Yamada et al, 2002). The present study investigated effects of acute hyperglycemia on brain cholinergic functions, oxidative stress and protein expression of BDNF on cognitive functions in diabetes mellitus.
2. Materials and Methods
- Laboratory Animal Twenty male adult (145.73±6.18g) Sprague-Dawley albino rats were obtained from the Laboratory Animal Centre of College of Medicine of the University of Lagos, Lagos. They were kept 6 rats per cage, at 28±2°C. The rats were housed in plastic cages and fed commercial diet and water ad libitum for 14 days of acclimatization. Care of all animals was as in accordance with the National Law on Animal Care and use (Zimmerman, 1983). The rats were allowed to acclimatize for 30 days during which they were fed with rat chow and water ad libitum, and weighed every other day. Induction of Diabetes MellitusStreptozotocin (STZ), was obtained from Sigma chemicals Co., St Louis, MO, USA, and- dissolved in 0.01M citrate buffer, pH 4.5. Diabetes was then induced intra-peritoneally with 65mg streptozotocin per kilogram body weight of each rat. Six male Sprague-Dawley rats from each cage, were left to fast overnight. Fasting blood glucose level of the rats was determined after which diabetes was confirmed to be present.Training Apparatus and Procedures: Shuttle Box AcquisitionThe apparatus used for training rats in order to assess their short-term and long-term memory was the shuttle box (Halas et al., 1983, Ebuehi and Akande, 2009). The shuttle box consisted of two wooden compartments of identical dimensions (28 by 15 cm).The floor consisted of 6 mm diameter wire rods ( spaced 1.7 cm apart at the centre) through which 1.5 mA of scrambled foot shock was administered. The rods were connected to a set down transformer with a regular dimmer which could be switched on and off to deliver an instant scrambled foot shock to either compartment. A miniature lamp was in the ceiling of the first compartment. The floor was divided into two equal compartments by a wooden door that the mouse crossed to avoid or escape foot shock. The door could be raised to permit entry of the rats into any of the two compartments. The training or learning task commenced after rats were acclimatized. (Ebuehi, 2012).At the beginning of each trial, the light was turned on in the compartment. At the 1st day of training, all rats were placed in the shuttle box and allowed to have access to both the light and dark compartment for a period of 1 h. On day 2 of training, rats from each group were placed in the illuminated compartment and then, 30 sec later the door was raised. The dark compartment indicated a "safer" compartment in which the mouse would not receive foot shock. To avoid foot shock and the light compartment, the rat had to cross into the safer compartment within 5s. Failing to do so resulted in foot shock. The inter-trial intervals were randomized at 30, 45, and 60s. For each rat, the daily training session consisted of 2 trials. Such training trials were conducted for about 5 days. The measure of acquisition was the number of avoidance responses per day and the number of mice making about 70% avoidances in a block of 2 trials. The learning procedure was repeated on days 3, 4 and 5. For short-term testing, 24h after training (day 2), each rat from the 2 groups was placed in illuminated chamber and 30 sec later the door was raised, and the time spent in the light compartment before entering the dark compartment was recorded. Learning skills acquired on days 5 depicted long-term memory (Ebuehi and Akande, 2009, Ebuehi, 2012).Collection of Plasma and OrgansThe rats were sacrificed by decapitation, after the Shuttle box acquisition had been completed. Brain, pancreas, liver and blood of each rat were collected using sample bottles and heparinized bottles respectively. Blood samples were immediately centrifuged at 15000 g for 10 min and plasma was collected. The excised organs were preserved under ice. The brain, pancreas and liver tissues were homogenized separately with 5 volumes of ice-cold 50mM Tris-HCl (pH 7.4) containing 30 mM sucrose. The homogenate was centrifuged at 15,000g for 30 min to remove nuclei and cell debris. The plasma was used for determination of total protein and C-peptide levels, while the resulting supernatant of the tissue homogenate was used for determination of anti-oxidant status, acetyl cholinesterase activity, acetylcholine level and BDNF protein expression. Histopathology of brain, liver and pancreasThe method of Baker and Silverton (1985) was employed for the processing of the tissue histology.Determination of Plasma C-Peptide levelThe direct immuno-enzymatic method was used to assay for the plasma C-peptide level in rat. The micro plate wells were formatted for each calibrator, control and specimen to be assayed. 50µl of both standard and sample were placed in separate micro-plate wells. Thereafter, 100µl of the conjugate was added. The set-up was incubated at 26°C for 2h. The content was removed from each well. The wells washed 3-times with 300µl of dilute wash solution. 100µl of TMB substrate was added separately to blank, standard, and sample. This was again incubated at 26°C for 15min in the dark. The micro plate was shaken gently and the absorbance read at 450nm against blank.Determination of Total ProteinThe method of Lowry et al., (1951) was used for protein determination using Bovine Serum Albumin (BSA) as standard. This method is based on the reaction between aromatic amino acids residues of the protein in the sample and phosphomolybdic-phosphotungestic acid present in the Folin-Ciocalteu reagent. The amount of protein in the sample is estimated by measuring absorbance at 750nm. The standard curve of Bovine serum albumin is prepared.Determination of Catalase (CAT) ActivityCatalase activity was determined according to the method of Sinha (1971) which measures the initial rate of H2O2 decomposition. This method is based on the reduction of dichromate in acetic acid to chromic acetate when heated in the presence of H2O2 with the formation of perchromic acid as an unstable intermediate. The chromic acetate produced is measured colorimetrically at 570–610nm and used as an index for estimating H2O2 decomposition and catalase (CAT) activity.Determination of Superoxide Dismutase (SOD) ActivityThe SOD activity was determined by the method of Misra and Fridowich (1972). The SOD assay is determined based on the ability of superoxide dismutase to inhibit the auto-oxidation of epinephrine at pH 10.2 with an increase in absorbance at 480nm. This makes the reaction a basis for simple assay for SOD. Determination of Reduced Glutathione Concentration The reduced glutathione (GSH) level was determined using Ellman’s reagent, 5,5'-dithio-bis-2-nitrobenzoic acid (DTNB) as described by Sedlak and Lindsay (1968) and Jollow et al., (1974). This method is based on the development of a relatively stable yellow complex formed as a result of reaction between Ellman’s reagent and free sulphydryl groups. The chromophoric product, 2–nitro–5– thiobenzoic acid, resulting from the reaction of Ellman’s reagent with GSH possesses a molar absorption at 412nm. The absorbance of this complex at 412nm is proportional to the level of GSH in the sample.Determination of Lipid PeroxidationThis was assayed by measuring the thiobarbituric acid (TBA) reactive products present in the test sample using the procedure of Vashney and Kale (1990) and expressed as micro molar of malondialdehyde (MDA)/g tissue. The assay was based on the reaction of chromogenic reagent with MDA (end product of lipid peroxidation) under acidic condition to yield a stable pink chromophone with maximum absorbance at 532nm. The MDA level was calculated according to the method of Adam–Vizi and Seregi (1982). Acetylcholine AssayThe Enzylite Acetylcholine Assay Kit by Assay Biotech Company USA was used for plasma acetylcholine determination. Acetyl cholinesterase catalyzes the reaction of acetylcholine to choline. Choline then gets oxidized to H2O2 by choline oxidase. In the presence of Horsedash peroxidase (HRP), the H2O2 reacts with a specialized fluorescent probe in a 1:1 stoichiometry to generate the red fluorescent oxidation product, resorufin. Resorufin has fluorescent excitation and emission maxima of approximately 571nm and 585nm and absorbance maxima at 570nm. 50ul of each sample/standard was added to the 96-wells flat bottom micro plate.The standard and sample were run in duplicate. 50ul of the working solution was added to the plates containing the standard, controls and test samples. It was incubated for 15min, while being protected from light. The plate was read using Emax micro plate reader at absorbance of 570nm and the concentration was extrapolated from standard curve plotted.Determination of Acetylcholinesterase ActivityThe method of Elman et al., (1961) was used for determination of acetyl cholinesterase (AChE) activity. Aliquots of the homogenate (0.4ml) were measured out and poured into a cuvette containing 2.6ml of phosphate buffer (0.1 M; pH 8.0) and 100µl of DTNB. The content inside the cuvette was mixed thoroughly and the absorbance was read at 412nm. The basal reading was then recorded when the absorbance had reached a stable value. 20µl of the substrate (acetyl thiocholine) was added and the change in absorbance recorded for 10 min and measured every 2 min. The change in absorbance per minute was determined. The acetylcholinesterase activity is measured by providing an artificial substrate acetyl thiocholine (ATC). Thiocholine; released because of the cleavage of ATC by AChE reacts with the – SH containing compound - 5,5 dithiobis- (2-nitrobenzoic acid) DTNB, which is reduced to thionitrobenzoic acid, a yellow colored anion with a maximum absorption of 412nm. The concentration of thionitrobenzoic acid is detected using a UV spectrophotometer and this is taken as a direct estimate of AChE activity. Western Blotting The brain, pancreas and liver were used for Western blotting analysis. Samples were kept cold and steps performed on ice. A small sample of fresh, unfixed tissue (brain, liver and pancreas) was finely chopped using a sharp scalpel. The chopped tissue was homogenized in a minimum volume of lysis buffer (with protease inhibitor mixture). The sample was centrifuged for 15 min at 10,000 x g to pellet solid debris. The supernatant (containing solubilized proteins) was obtained and the pellet (intact Cells/cell debris) was discarded. The supernatants were directly used for SDS-PAGE and Western blotting analysis. The detailed protocol has been described according to the method of Burnette (1981). 15% polyacrylamide gradient gels were used to detect full-length BDNF. Rabbit polyclonal anti-BDNF antibodies were from Signalway Antibody. Statistical AnalysisStatistical analysis was performed with the Statistical Package for the Social Science (SPSS, Chicago, IL, USA) program, version 16.0. Data were analyzed by employing Student’s t-test. Data were expressed as Mean ± SEM. Mean values were assessed for significance by Student’s t-test at P < 0.05. The protein bands (Western blots) obtained were analyzed for protein intensity using Gel analyzer version 2010a.
3. Results
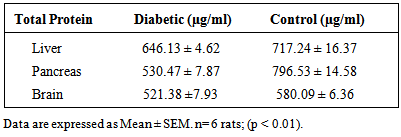
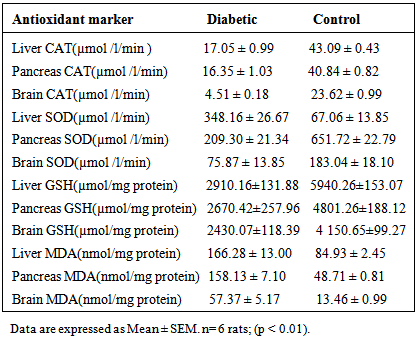
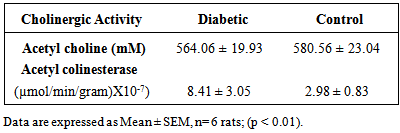
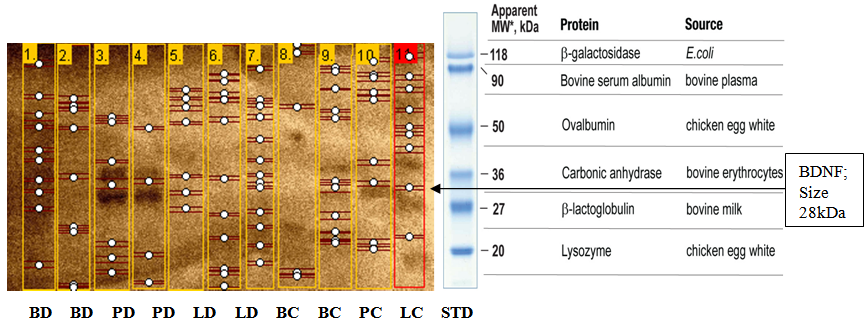
- Results of body weight of diabetic and control rats are shown in Fig. 1. The body weight of diabetic rats compared to the control significantly decreased (p<0.05). The fasting blood glucose of both control and diabetic rats are shown in Fig. 2. The concentration of glucose in diabetic group was significantly higher than the control group (Fig. 2). The plasma C-peptide level of diabetic rats significantly decreased compared to the plasma C-peptide level of the control rats (Table 1). The Shuttle box acquisition training in streptozocin - induced diabetic (STZ-D) and control rats are shown in Fig.3. The STZ-diabetic rats showed much slower rate of learning acquisition in terms of the time taken to cross over from the light to the dark phase in Shuttle box than controls. On days 4 and 5, difference in number of responses reached statistical significance (Fig. 3).The concentration of total protein in diabetic and control rats is presented in Table 2. The total protein level of diabetic rats was significantly lower than in the control rats (Table 2). Antioxidant enzyme markers in the pancreas, brain and liver of diabetic and control rats are shown in Table 3. The antioxidant enzyme activities of catalase, superoxide dismutase, and reduced glutathione level in the organs of the diabetic rats were significantly (p <0.01) reduced compared to the control rats. However, malondialdehyde (MDA) levels present in the organs of the diabetic rats, were significantly (p <0.01) elevated compared to control rats (Table 3).The acetyl choline concentration and acetyl cholinesterase activity of the diabetic rats and control rats are presented in Table 4. Acetylcholine concentration decreased significantly in the brains of diabetic rats. In contrast, the acetylcholinesterase activity in the brains of diabetic rats increased significantly compared to the control (Table 4).The Western blot of full length BDNF (Brain Derived Neurotrophic Factor) in diabetic and control rats is presented in Fig.4. The BDNF was detected in both diabetic and control rats (Fig. 4), but had decreased protein expression in the brain, pancreas and liver of the diabetic rats compared to control (Fig. 5).Diabetic rats had significant decrease in the body weight, compared to that of control rats (Fig.1).
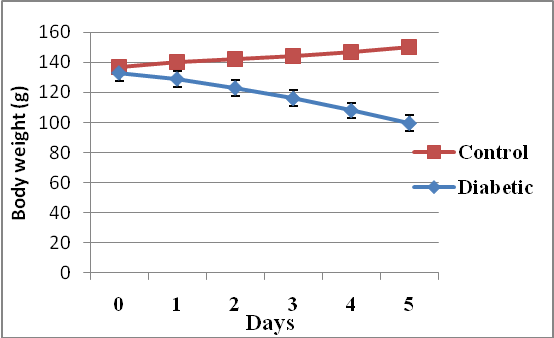 | Figure 1. Body weight changes of diabetic and control rats after the induction of type II diabetes mellitus (Data are expressed as mean ± SEM. n= 6 rats; (P < 0.05) |
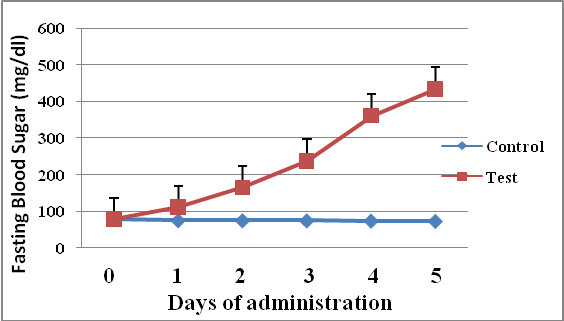 | Figure 2. Fasting blood glucose levels of diabetic and control rats (Data are expressed as mean ± SEM, n= 6 rats; (p < 0.01) |
|
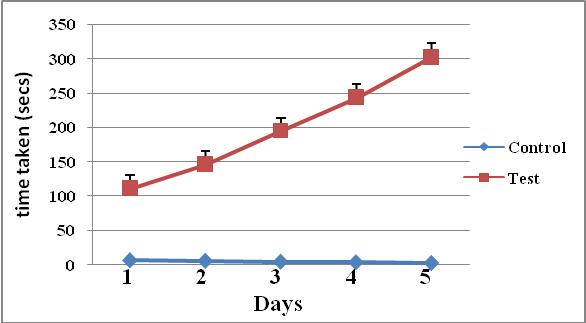 | Figure 3. Learning skill test using Shuttle box acquisition of streptozocin-induced diabetic and control rats (Data are expressed as Mean ± SEM, n = 6 rats; p < 0.05) |
|
|
|
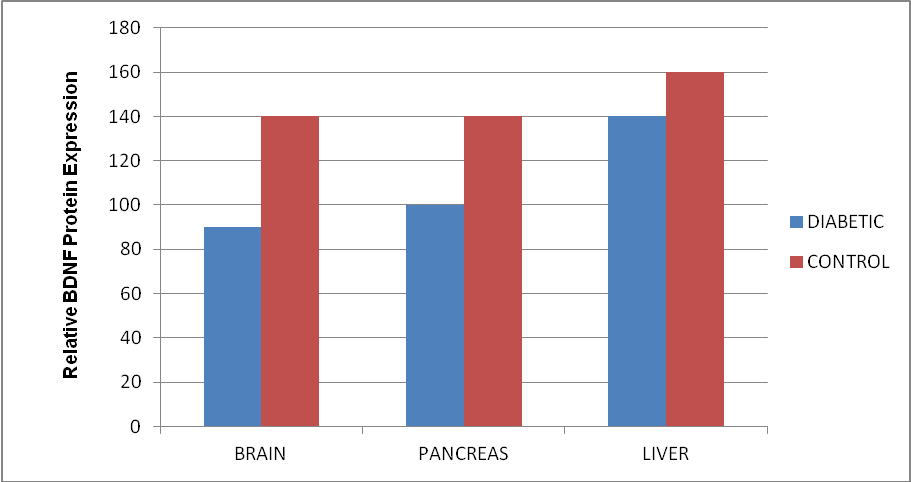 | Figure 5. Relative BDNF protein expression of brain, pancreas and liver of diabetic and control rats. Data are expressed as mean.for the control brain, diabetic brain, daibetic liver and the pancreas |
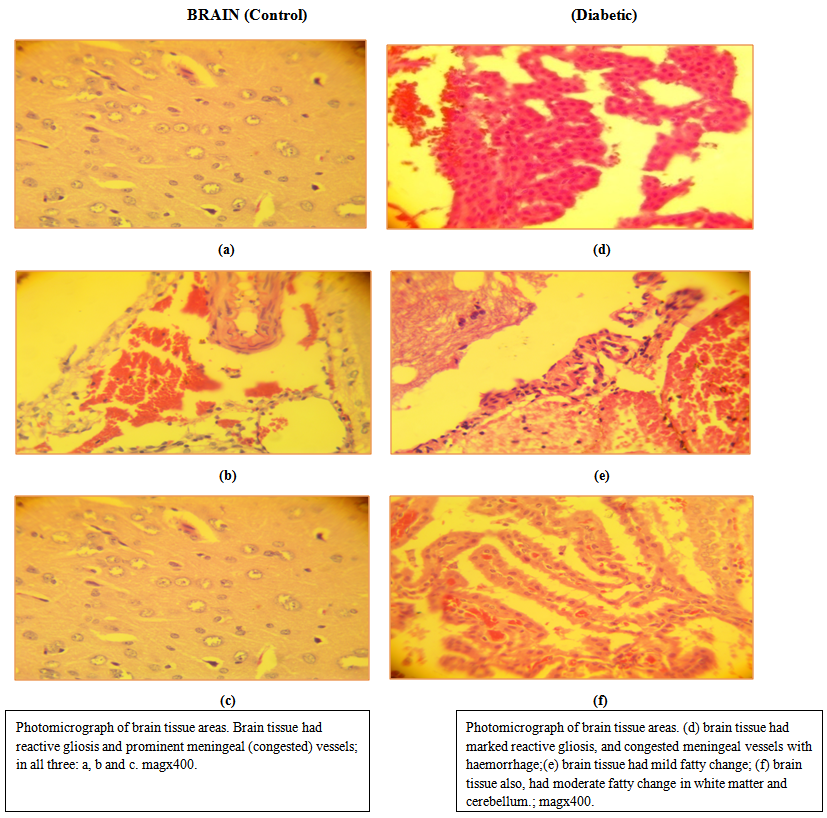 | Figure 6. Histopathology of the brain of control and streptozotocin induced diabetic rats |
4. Discussion
- The STZ-administered rats had significantly increased blood glucose levels than the control as shown similarly in previous reports by Akbarzadeh et al. (2007), which indicates that the rats were diabetic. There was a significant decrease in the circulating plasma C-peptide level of diabetic rats compared to control, which suggest that the diabetic rats may have suffered from plasma C-peptide deficiency. Since C-peptide and insulin are released in equimolar amounts from the β-cells of the pancreas, the measurement of C-peptide has been used as a marker of β-cell function and an index of insulin secretion. In view of this it can be said that the diabetic rats would have been insulin deficient.Although the mechanisms underlying CNS dysfunction are not clear, one of the contributing factors could be insulin deficiency with concomitant C-peptide deficiency. Thus in the absence of sufficient amount of insulin, the brain cells will exhibit severe hypoglycemic condition. This observation is also in agreement with Gerozissis et al., (2001), Zhao and Alkon (2001, who have demonstrated that insulin exerts additional modulatory roles on brain functions such as feeding, learning, and memory. Glucose is the main brain energy supply for the maintenance of the nervous system, thus in this study, the deficiency of glucose in the cells will trigger neuronal injury. This could be as a result of impaired energy metabolism in neurons which will induce the production of increased amount of free radicals in the diabetic rats, which in turn also will induce neuronal cell damage, due to the presence of increased oxidative stress in the brains of the diabetic rats. The existing presence of this stress is due to the significantly reduced levels of antioxidants observed in the diabetic rats. The above explanation confirms the findings of Aksenov et al. (2001), Bunsey et al. (1996), Regan et al. (2001), Suzuki and Clayton (2000) that oxidative damage to various brain regions constitute in the long term complications, morphological abnormalities and memory impairments.In addition, in a state of insulin deficiency as observed in this study, the stimulatory effect of the actions of insulin on protein synthesis will be disturbed, thus leading to decreased or abnormal levels of total proteins. This finding of decreased total protein levels in diabetes is in agreement with the reports of O’neil et al, (2010); who reported that in the absence of insulin, protein synthesis is not favored. This finding was also confirmed by a significant decrease in plasma albumin levels and antioxidant enzyme activities in the diabetic rats compared to the control rats. Central cholinergic activity was studied in STZ-induced diabetic rats using acetylcholine and acetyl cholinesterase (AChE) as markers. The results showed an increase activity of AChE in the brains of diabetic rats when compared to control. Thus it could be said that the increase in AChE activity was caused by the experimental diabetes because of the much reduced levels of glucose (energy) in the brain cells. This therefore could, result in decreased efficiency of cholinergic neurotransmission, due to decreased levels of acetylcholine in the synaptic cleft in the brains of the diabetic rats. This therefore could contribute to the progressive cognitive impairment observed in diabetic rats. Therefore, the acetylcholine deficiency observed in the diabetic rats is supported by increased AChE activity in the diabetic rat brain (Sonkusare et al., 2005). Additionally, the normal roles of acetylcholine cannot be achieved; as such learning deficits, cognitive dysfunction in the diabetic rats, becomes obvious. These results are in accordance with Kuhad et al. (2007) who reported a significant elevation in AChE activity in cerebral cortex from STZ-induced diabetic rats.The protein expression studies of brain derived neurotrophic factor (BDNF) revealed a down regulation in the brain regions of the diabetic rats. In addition, BDNF protein expression was observed to be down regulated in the liver and pancreas of the diabetic rats compared to the control rats. The results of the present study is in agreement with previous report of Nitta et al.(1999), who showed a decrease in the expression of this neurotrophin in diabetes. The reduction in antioxidant defense system (decreased antioxidant levels) observed in this study could have caused an increased ROS-induced neuronal injury, in the diabetic rats. (Facchinetti et al., 1998). The increased oxidative stress which occurred as a result the pre-existing diabetes in the rats could cause a reduction in the expression BDNF, and this could have contributed to the memory impairments observed in the diabetic rats.Promising research is emerging around – BDNF, in cognitive dysfunction associated neurological disorders. In the brain, BDNF promotes the optimum communication between neurons by enhancing “plasticity” at the synapse (Blurton-Jonesa et al., 2009). This is a very important mechanism underlying learning and memory in the adult CNS. The adaptive capacity by the brain is reflected in the ability to integrate information and experiences, and through higher cognitive processing, anchor them into learning and memory formation. The synapse is a critical locus in this adaptive capacity. BDNF plays an important role in the survival of neurons, their growth (axons and dendrites), and the formation and function of the synapse. (Blurton-Jonesa et al, 2009) Without sufficient BDNF and other neurotrophic factors, neurons die (Yamada et al, 2002). Cholinergic transmission in the brain cortical and hippocampal regions plays a fundamental role in memory (Hasselmo et al, 2006).
5. Conclusions
- Data of the study report that diabetes mellitus resulted in down regulation of the protein expression of brain derived neurotrophic factor and reduction in brain acetylcholine, plasma C-peptide, total plasma protein levels, acetyl cholinesterase activity and antioxidant status in rats. Therefore, diabetes mellitus may cause some degree of neuronal injury and subsequent impairment of learning, memory and cognitive functions.
ACKNOWLEDGEMENTS
- The authors are grateful to Mrs. Adenike Fowora and Mr. Babatunde James for their laboratory assistance in the Western blotting technique used in the study and to the National Institute of Medical Research, Yaba, Lagos, where a part of the assay was carried out.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML