| [1] | Berrendero, F. & Maldonado, R. (2002). Involvement of the opioid system in the anxiolytic-like effects induced by Delta (9)-tetrahydrocannabinol. Psychopharmacology 163(1): 111 – 117. |
| [2] | Benson, M. & Bentley, A. M. (1995). Lung disease induced by drug addiction. Thorax, 50: 1125 – 1127. |
| [3] | Bindra, D. & Thompson, W. R. (1953). An evaluation of defecation and urination as measures of fearfulness. Journal of comparative and physiological psychology, 46: 43-45. |
| [4] | Bird, C. M. & Burgess, N. (2008). The hippocampus and memory; Insights from spatial processing. Nature review neuroscience 9: 182. |
| [5] | Blanchard, D. C., Griebel, G., & Blanchard, R. J. (2001). Mouse defensive Behaviors: Pharmacological and behavioral assays for anxiety and panic. Neuroscience and biobehavioral reviews, 25: 205-218. |
| [6] | Bloom, F. & Lazerson, A. (1988). Brain, Mind and Behaviors (2nd ed.) New York, W. H. Freeman. P. 300. |
| [7] | Bourin, M. & Hascoët, M. (2003). The mouse light/dark box test. European journal of pharmacology, 463: 55-65 |
| [8] | Brown, R. E., Corey, S. C., & Moore, A. K. (1999). Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behavior genetics, 29: 263-271. |
| [9] | Budney, A.J., Hughes J.R., Moore B.A. & Vandrey R.G., (2004). A review of the validity and significance of the cannabis withdrawal syndrome. American journal of psychiatry 161: 1967–1977. |
| [10] | Callaway J. C (2004). Hemp seed as nutritional resource. A review: Euphytica 14: 65 – 72. |
| [11] | Costall, B., Jones, B.J., Kelly, M.E., Naylor, R.J. & Tomkins, D.M. (1989). Exploration of mice in a black and white test box: Validation as a model of anxiety. Pharmacology, biochemistry and behavior, 32:777-785. |
| [12] | Coulter. C., Taruc, M., Tuyay, J. & Moore C. (2009). Quantitation of tetrahydrocannabinol in hair using immunoassay and liquid chromatography with tandem mass spectrometric detection. Drug test analysis 1: 234–239. |
| [13] | DiCarlo E. & Izzo, S. (2003). Cannabinoids for gastrointestinal diseases: Potential Therapeutic Applications. Expert opinion on investigational drugs 12: 39-49. |
| [14] | D'Hooge, R., and P. P. De Deyn. (2001). Applications of the Morris water maze in the study of learning and memory. Brain Research. Brain research reviews 36 (1):60-90. |
| [15] | D’Souza, D. C., Perry, E., MacDougall, L., Ammerman, Y., Cooper, T., Wu YT, Braley G, Gueorguieva R, Krystal, J. H. (2004). The psychotomimetic effects of intravenous delta-9- tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 29:1558 –1572. |
| [16] | Dos Santos, Michel David; Almeida, Maria Camila; Lopes, Norberto Peporine; De Souza, Gloria Emilia Petto (2006). Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biological and pharmaceutical bulletin 29(11):2236-40. |
| [17] | Duck, P.; Katsuyuki, S. (2004). The frontal cortex & working memory. Physiology & Brain imaging. Current opinion neurobiology: 14: 163. |
| [18] | Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. (1999) The hippocampus, memory and place cells: is it spatial memory or a memory space? Neuron, 23, 209–226. |
| [19] | Elphick, M. R.; Egertova, M. (2001). The neurobiology and evolution of cannabinoid signaling. Philosophical transactions of the royal society B: Biological sciences 356 (1407): 381–408. |
| [20] | Elsohly, Mahmoud (2007). Marijuana and the cannabinoids. Humana Press. P.8 |
| [21] | Elsohly, M.A., E. Harland, J.C. Murphy, P. Wirth, and C.W. Waller. (1985). Cannabinoids in glaucoma: a primary screening procedure. Journal of clinical pharmacology 21: 472S-478S. |
| [22] | Elsohly, M. A., E.C. Harland, D.A. Benigni, and C.W. Waller. (1984). Cannabinoids in glaucoma II: the effect of different cannabinoids on intraocular pressure of the rabbit. Current eye research. 3: 841-850. |
| [23] | Enna S.J., (1984). Role of gamma-amino butyric acid in anxiety. Psychopathology. 17 (Suppl. 1): 15–24. |
| [24] | Espejo, E. F. (1997). Effects of weekly or daily exposure to the elevated plus-maze in male mice. Behavioural brain research, 87: 233-238. |
| [25] | Eubanks, Lisa M.; Rogers, Claude J.; Beuscher, 4th; Koob, George F.; Olson, Arthur J.; Dickerson, Tobin J.; Janda, Kim D. (2006). "A Molecular Link between the Active Component of Marijuana and Alzheimer's Disease Pathology". Molecular pharmaceutics 3 (6): 773–7. |
| [26] | Fant, R (1998). "Acute and Residual Effects of Marijuana in Humans". Pharmacology biochemistry and behavior 60 (4): 777–84. |
| [27] | Fernandez, J. and Allison, B. (2004). Rimbonabant, Sanofi-Synthelabo. Current opinion in investigational drugs, 5: 430 – 435. |
| [28] | Fix James D. (2008) Basal Ganglia and the striatal motor system. (4th Ed.) Baltimore: Wulters Kluwer &LippinCott Williams & Wilkins pp. 274-281. |
| [29] | Fordyce, D., Clark, V., Paylor, R. & Wehner, J. (1995). Enhancement of hippocampally-mediated learning and protein kinase C activity by oxiracetam in learning-impaired DBA/2 mice. Brain research, 672, 170-176. |
| [30] | Fox, G., LeVasseur, R. & Faden, A. (1998). Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS Mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. Journal of neurotrauma, 16, 377-389. |
| [31] | Frick, K.M., Stillner, E.T., & Berger-Sweeney, J. (2000). Mice are not little rats: species differences in a one-day water maze task. Neuroreport, 11, 3461-3465. |
| [32] | Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, Martin-Santos R, Seal ML, O'Carrol C, Atakan Z, Zuardi AW, McGuire P. (2010). Modulation of effective connectivity during emotional processing by Delta (9)-tetrahydrocannabinol and cannabidiol. International journal of neuropsychopharmacology. 13(4): 421 - 32. |
| [33] | Gallagher, M. & Rapp, P. R. (1997). The use of animal models to study the effects of aging on cognition. Annual review of psychology. 48: 339 - 370. |
| [34] | Gallagher, M., Burwell, R. & Burchinal, M. (1993). Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behavioural neuroscience, 107: 618-626. |
| [35] | Genn RF, Tucci S, Marco EM, Viveros, M. P. & File, S. E. (2004). Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. Pharmacology and biochemical behavior 77: 567 – 573. |
| [36] | Germano, M. P., D’Angelo V., Monvello, R. Pergollizzi, S., Capasso, F., Capasso, R., Izzo, A. A., Moscow, N. & Depasquace R. (2001). Cannabinoids CB1 medicated inhibition of stress induced gastric ulcer in rats. Naunyn schmuderbergs archives pharmacology. 363: 241 – 244. |
| [37] | Gosh, M. N. & Schild, H. D. (1958). Continuous recording of acid secretion in rats. British journal of pharmacology, 13; 54 – 61. |
| [38] | Graham, T. (1998). Fertility complications due to drug intake. British journal of fertility 189: 204 – 216. |
| [39] | Grimmer, Heidi R; Parbhoo, Veena; Mc Grath, Robert, M. (1992). Antimutagenicity of polyphenol-rich fractions from Sorghum bicolor grain. Journal of the science of food and agriculture 59(2): 251-256. |
| [40] | Grotenhermen, Franjo (2007). The Toxicology of Cannabis and Cannabis Prohibition. Chemistry & biodiversity 4 (8): 1744–69. |
| [41] | Jones, R. T.; (1983). Cannabis tolerance, dependence and health hazards. (Eds. K.O. Fehr & Kalant, H.) Toronto: Addiction, 23: 825-55. |
| [42] | Jose’ Alexandre de Souza Crippa, Antonio Waldo Zuardi, Griselda E.J.; Lauro Wichert-Ana, Richardo Guarnieri, Lucas Ferrari, Paulo M Azevedo-Marques, Jaime Eduardo Cecilio Hallak, Philip K. McGuire and Geraldo Filho Busatto (2003). Effects of Cannabidiol (CBD) on Regional Cerebral blood flow. Neuropsychopharmacology, 29(2): 417 - 426. |
| [43] | Joy J. E., Watson S. J., Jr., and Benson J. A., Jr. (1999). Marijuana and Medicine: Assessing the Science Base. Washington, D.C.: National Academy of Sciences Press. |
| [44] | Kalant H. K & Roschlau H.E. (1998). Principles of medical pharmacology (6th Ed) pp. 373Maykut, 1985-375. |
| [45] | Kandel, R. (2001). Molecular Biology of memory storage”. The dialogue between genes & synapses. Science 294: 1030- 1038. |
| [46] | Kim, S., Lee, S., Ryu, S., Suk, J-g. & Park, C. (2002). Comparative analysis of anxiety-related behaviors in four inbred mice. Behavioural processes, 60:181-190. |
| [47] | King S.A. (2008). Cannabinoids and pain. Psychiatric times 25(2). |
| [48] | Kirkham, M. B. (2006). Cadminim in plants, on polluted soils: Effect of soil factors hyper accumulation and amendment. Geoderma Vol. 137 Issues 1 – 3, pg. 19 – 32. |
| [49] | Klapdor, K. & van der staay, F.J. (1996). The Morris water-escape task in mice: strain differences and effects of intra-maze contrast and brightness. Physiology of behavior 60: 1247-1254. |
| [50] | Klein, T.W., Friedman, H. and Specter, S. C. (1998). Marijuana, immunity and infection. Journal of neuroimmunology. 83:102-115. |
| [51] | Klein TW, Lane B, Newton CA, Friedman H. (2000). The cannabinoid system and Cytokine network. Experimental biology & medicine 225:1-8 |
| [52] | Manzanares, J., Corchero, J., & Fuentes, J. (1999). Opioid and cannabinoid receptor-mediated regulation of the increase in adrenocorticotropin hormone and corticosterone plasma concentrations induced by central administration of delta (9)-tetrahydrocannabinol in rats. Brain research, 839(1): 173 – 179. |
| [53] | Matsuda, L. A., Lolait S. J., Brownstein M. J. (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature, 346: 561-564. |
| [54] | Mattes, R.D., Engelman, K., Shaw, L.M., (1994). Cannabinoids and appetite stimulation. Pharmacology and biochemistry of behavior, 49: 187-195. |
| [55] | Maykut, M. O. (1985). Health consequences of acute and chronic marijuana use. Progress inneuropsychopharmacology and biological psychiatry, 9: 209 – 223. |
| [56] | McDonald, R.J. & White, N.M. (1994). Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behavioral neural biology, 61: 260-270. |
| [57] | McKim, W. A. (2002). Drugs and Behavior: An introduction to behavioral pharmacology. (5th Ed.). Upper Saddle River, Prentice Hall. p. 400. |
| [58] | McLaughlin, M. A., & Chiou, G. C. (1985). A synopsis of recent developments in antiglaucoma drugs. Journal of ocular pharmacology. 1:101-121. |
| [59] | Mechoulam, R., Petersa, M., Murillo-Rodriguez, E. & Hanus, L. O. (2007). Cannabidiol: recent advances. Chemistry and biodiversity, 4: 1678 - 1692. |
| [60] | Merritt, J. C., Crawford, W. J., Alexander, P. C., Anduze, A. L. & Gelbart, S. S. (1980). Effect of marijuana on intraocular and blood pressure in glaucoma. Ophthalmology, 87: 222-228. |
| [61] | Misner D. L. & Sullivan J. M. (1999). Mechanism of Cannabinoid effects on long term potentiation and depression in hippocampal CA1 neurons. Journal of neuroscience, 19: 6795 - 805. |
| [62] | Moor, R. A. & McQuay, H. (2005). Prevalence of opoid adverse events in chronic pain, system review of randomed trials of oral opoids. Arthritis research and therapy, 7(5): R1046-51. |
| [63] | Moreira, F. & Lutz, B. (2008). The endocannabinoid system: emotion, learning and addiction. Addict biology, 13(2): 196–212. |
| [64] | Morgan, D. G., Diamond, D. M., Gottschall, P. E., Ugen, K. E., Dickey, C., Hardy, J. D., Duff, K., Jantzen, P., DiCarlo, G., Wilcock, D., Connor, K., Hatcher, J., Hope, C., Gordon, M. N., & G. W. Arendash. (2000). A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature, 482: 982 - 986. |
| [65] | Navarro M, Fernandez-Ruiz JJ, de Miguel R, Hernandez ML, Cebeira M, Ramos JA (1993) An acute dose of delta 9-tetrahydrocannabinol affects behavioral and neurochemical indices of mesolimbic dopaminergic activity. Behavioral brain research. 57: 37–46. |
| [66] | Nemeroff C.B. (2003). The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacology bulletin. 37(4): 133–146. |
| [67] | Novak J, Zitterl-Eglseer K, Deans SG, Franz CM (2001). Essential oils of different cultivators of Cannabis sativa and their antimicrobial activity. Flavour and fragrance journal 16(4): 259-262. |
| [68] | Noyes, R., S.F. Brunk, D.A. Avery, and A.C. Canter. 1975. The analgesic properties of A-9-tetrahydrocannabinol. Clinical pharmacology and therapy. 18: 84-89. |
| [69] | Nunn, J. A., E. LePeillet, C. A. Netto, H. Hodges, J. A. Gray, and B. S. MeLDrum. (1994). Global ischemia: hippocampal pathology and spatial deficits in the water maze. Behavioral brain research 62 (1):41-54. |
| [70] | O'Keefe, J. (1976). Place units in the hippocampus of the freely moving rat. Experiment on neurology.51:78-109. |
| [71] | Okwari, O. O. (1999). Pharmacological properties of Dombeya buttneri in experimental animal PG.D Thesis. Department of Physiology, University of Calabar, Calabar- Nigeria. |
| [72] | Onaivi ES, Green MR & Martin BR (1990). Pharmacological characterization of cannabinoids in the elevated plus maze. Journal of pharmacology and experimental therapeutics, 253: 1002-1009. |
| [73] | Osborne Geraint and Curtis Forgel (2007). The normalization of marijuana used by adult Canadians users. International journal of crime, criminal justice and law 2(2): 201 – 225. |
| [74] | Pate, D. W. (1994). Chemical ecology of cannabis. Journal of international hemp association. 2: 29, 32 – 37. |
| [75] | Pertwee, R. G. (2006). The pharmacology of cannabinoid receptors and their ligands: An overview. International journal of obesity 30: S13–S18. |
| [76] | Pertwee, R. G.; Thomas, A. Stevenson, L.A (2007). The psychoactive plant cannabinoid,delta-9-tetrahydrocannabinol, is antagonized by delta-8-& delta-9-tetrahydrocannabivarin in mice in vivo. British journal pharmacology 150(5):586-94. |
| [77] | Phan K, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. 2008. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. Journal of neuroscience 28(10): 2313–2319. |
| [78] | Pickens, JT (1981). Sedative activity of cannabis in relation to its delta'-Trans-tetrahydrocannabinol and cannabidiol content. British journal of pharmacology 72 (4): 649–56. |
| [79] | Raud, S., Innos, J, Abramov, U., Reimets, A., Kõks, S., Soosaar, A., Matsui, T. & Vasar, E. (2005). Targeted validation of CCK2 receptor gene induces anxiolytic-like action in light-dark exploration, but not in fear conditioning test. Psychopharmacology, 181: 347-357 |
| [80] | Roy-Byrne M, Uhde T. 1988. Exogenous factors in panic disorder: Clinical and research implications. 49: 56–61. |
| [81] | Rudgley, Richard (1998). Lost civilizations of the Stone Age. New York. Free press. |
| [82] | Sameth, J. H. (2007). Drug abuse and dependence in Goldman L, Ausiello D. ed. Cecil Medicine 23rd ed. Philadelphia Pa! Saunders Elsevier Chap. 32. |
| [83] | Tournier M, Sorbara F, Gindre C, Swendsen J, & Verdoux H. (2003). Cannabis use and anxiety in daily life: a naturalistic investigation in a non-clinical population. Psychiatry research, 118: 1–8. |
| [84] | Trease, G. E. & Evans, W. C. (1989). A textbook of pharmacology (11th ed.). London: Bailliese Tindall. pp. 397 – 531. |
| [85] | Trullas, R., & Skolnick, P. (1993). Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology, 111: 323-331. |
| [86] | Tunving, K. (1987). Psychiatric aspects of cannabis use in adolescents and young adults. Pediatrician, 14(1–2): 83–91. |
| [87] | Turner, C. E., Bouwsma, O. J., Billets, Steve. & Elsohly, M. A. (1980). Constituents of Cannabis sativa: LXVIII – Electron voltage selected ion monitoring study of cannabinoids. Biological mass spectrometry, 7 (6): 247–56. |
| [88] | Van Os J., & Kapur, S. (2009). Schizophrenia. Lancet, 374(9690):635–45. |
| [89] | Van S. A., De Graaf, R., Acarturk, C., & Cuijpers, P. (2009). Psychological treatment of social anxiety disorder: A meta-analysis. Psychological medicine, 39(2):241-254. |
| [90] | Van T. K., Canfyn, M., Govaerts, C., Lenaerts, K., Piette, V. & Parmentier, F. (2004). Concentration of THC in cannabis in 2003. International Journal of Drug Policy, 21(4):271-75. |
| [91] | Viveros, M. P., Marco, E. M., & File, S. E. (2005). Endocannabinoid system, stress and anxiety responses. Pharmacology, Biochemistry & Behaviors, 81:331–342. |
| [92] | www.onlinepot.org/medical/immunesystem.htm. Marijuana and immune system. Retrieved, 8 October 2010. |
| [93] | Yan, Q. J., Asafo-Adjei, P. K., Arnold, H. M., Brown, R. E. & Bauchwitz, R. P. (2004). A phenotypic and molecular characterization of the fmr1-tm1Cgr Fragile X mouse. Genes, brain and behavior, 3:337-359. |
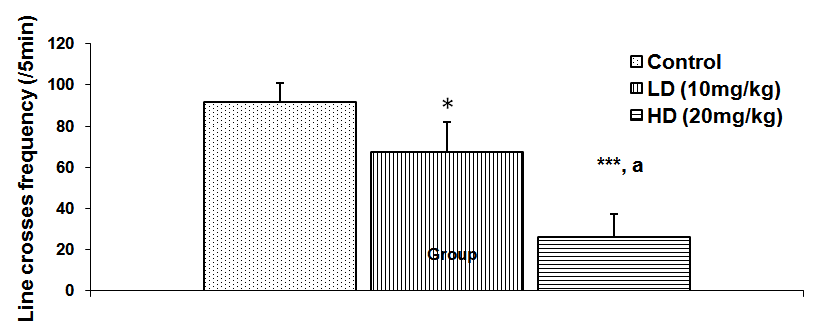
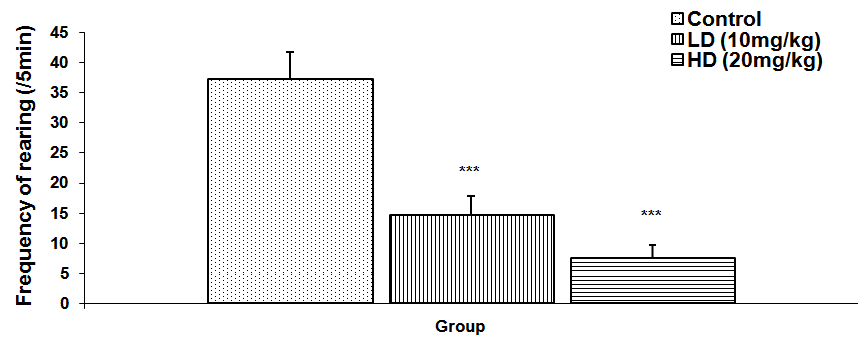
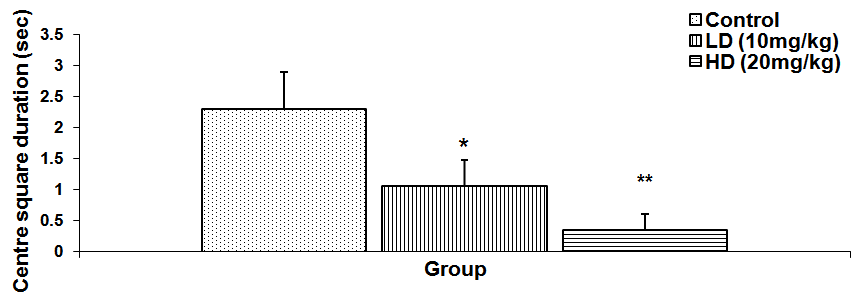
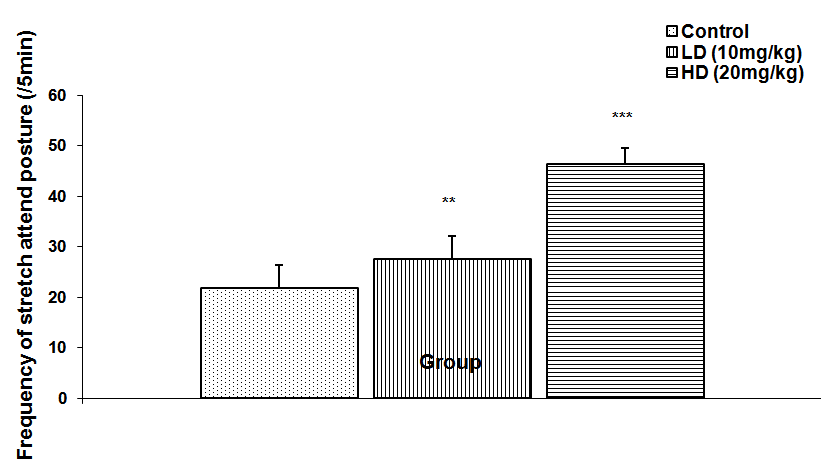
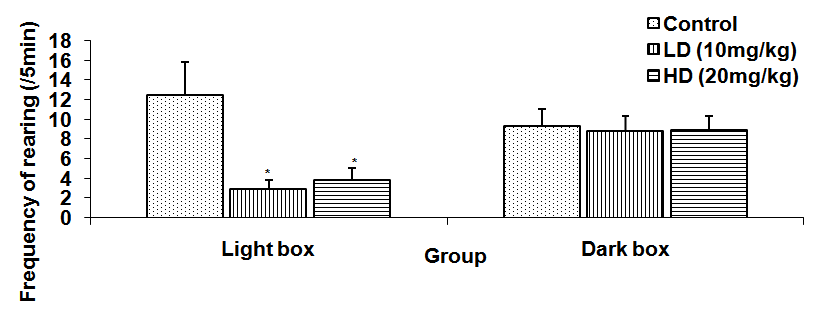
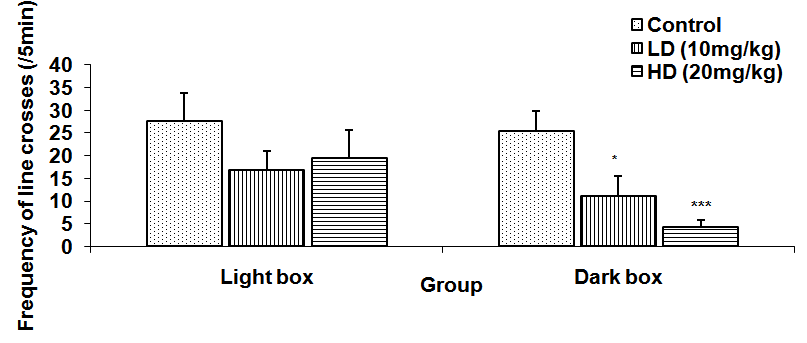
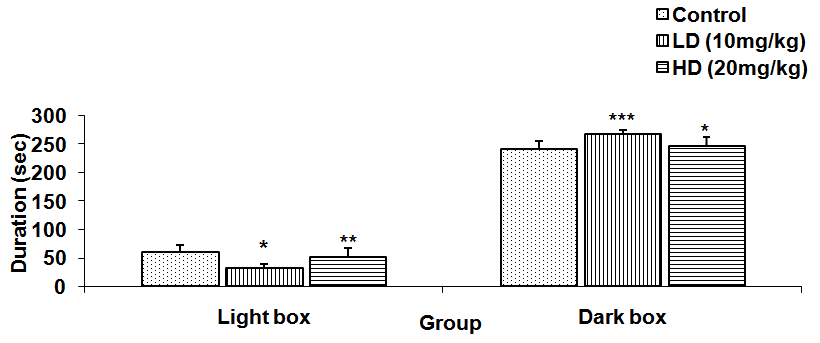
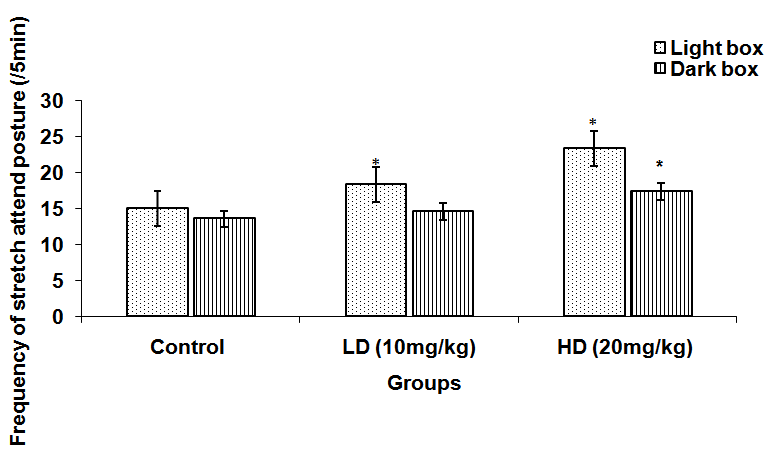
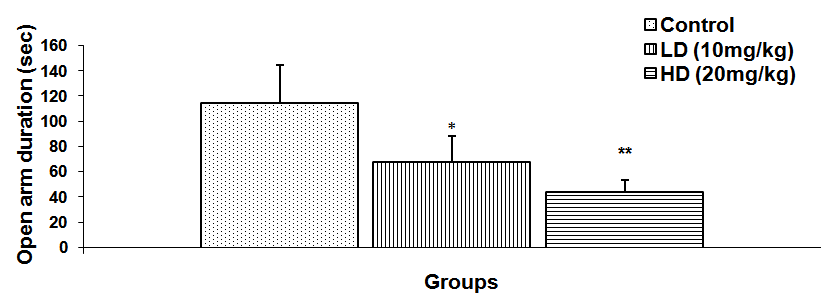
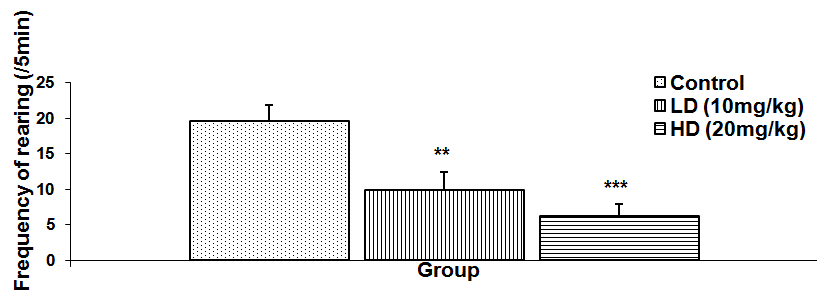
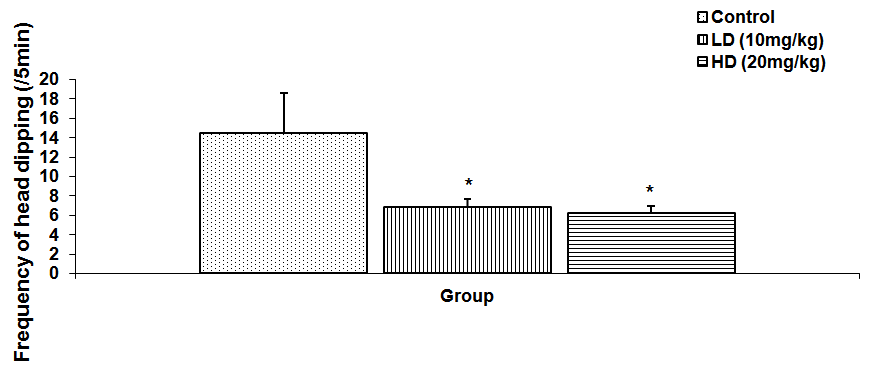
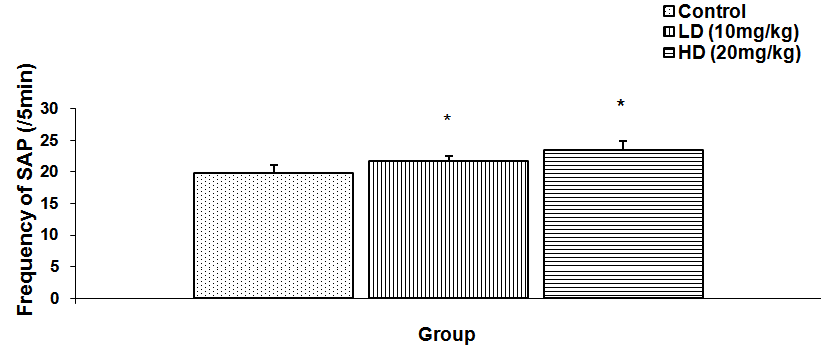
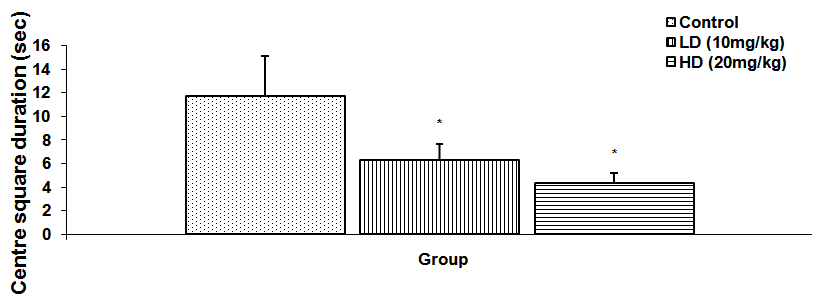
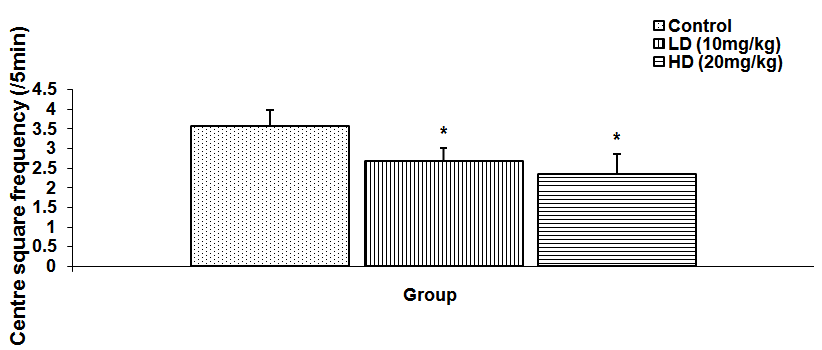
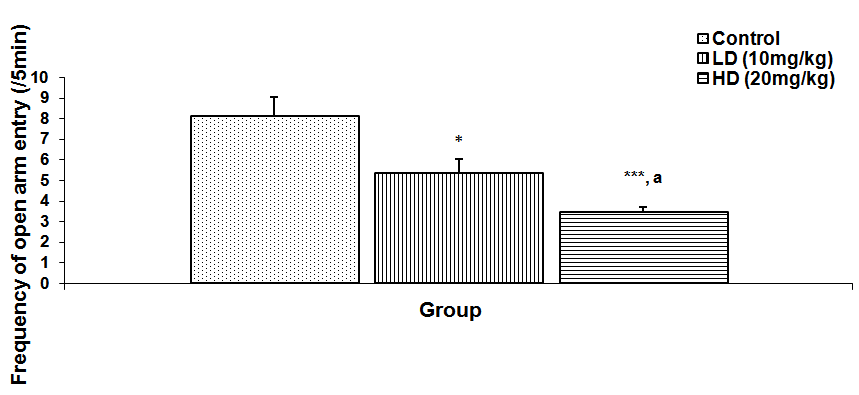
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML