-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2014; 3(1): 1-6
doi:10.5923/j.neuroscience.20140301.01
Quantitative Biometry of Body and Brain in the Grasscutter (Thryonomys swinderianus) and African Giant Rat (Cricetomys gambianus): Encephalization Quotient Implication
1Department of Veterinary Anatomy, College of Veterinary Medicine, University of Agriculture, Makurdi, Nigeria
2Department of Veterinary Physiology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
Correspondence to: O. Byanet, Department of Veterinary Anatomy, College of Veterinary Medicine, University of Agriculture, Makurdi, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Relationships between the brain and body weights were quantitatively examined in two important species African rodents to determine how accurate to estimate the former from the latter, and also the level of their intelligence using encephalization quotient (EQ). Twenty five animals, made up of 13 grasscutters (6 males and 7 females) and 12 African giant rats (6 males and 6 females) in each case were weighed and measured using a standard quantitative morphometric method. The results indicated that, for many of the behavioural repertoires of the grasscutter (GRC) and African giant rat (AGR), there were similarities in the gross features of their brains. The brain weight of the GRC was almost twice larger than that of the AGR (P < 0.0001), and significantly correlated positively with its body weight (r = 0.65, P < 0.01), whereas, the brain of the AGR correlated significantly with its EQ (r = 0.77, P < 0.003) and negatively with its body weight (r = -0.05). The mean EQ of the GRC (0.40) was significantly higher than that of AGR (0.19) (P < 0. 01). Also, the males mean EQ in the GRCs and females EQ in the AGRs were higher than their females or males counterparts, respectively. In conclusion, brain weight may be estimated accurately from body mass in the GRC. The GRC has a higher intelligence than the AGR, but both species have EQ values below average intelligence.
Keywords: Brain and body weights, T. swinderianus, C. gambianus, Encephalization quotient, Intelligence
Cite this paper: O. Byanet, T. Dzenda, Quantitative Biometry of Body and Brain in the Grasscutter (Thryonomys swinderianus) and African Giant Rat (Cricetomys gambianus): Encephalization Quotient Implication, Research in Neuroscience , Vol. 3 No. 1, 2014, pp. 1-6. doi: 10.5923/j.neuroscience.20140301.01.
Article Outline
1. Introduction
- The brain is one of the most important organs in the body because it controls so many body functions. Some neuroanatomical measures, including brain size, have been noted to scales allometrically to that of the body and deviations from this relationship were used to estimate an animal’s relative cognitive ability[1]. It has been stipulated that species with larger brain size in relation to body mass have greater ability to process and utilize complex information. For example, increased brain size has been linked to sensory specialization cognitive skills and motor functions[2, 3]. Also, brain size is said to be associated with memory storage and that memory and intelligence may be interrelated[1]. Also, it has been argued that large brain relative to body size could confer advantages to individuals in the form of behavioral flexibility[4]. Apart from mammals, several of these observations have also been documented in birds[5]. Hypotheses such as the brain-environment change have been put forward to explain such advantages of animal with larger brain[4]. In this hypothesis, it was explained that a large brain can have benefits in novel environments by facilitating adaptive behavioural response to unusual or novel ecological changes, through cognitive processes such as innovation, learning and decision making[6]. Another hypothesis is the cognitive buffer hypothesis, which suggests that large and more complex brain function may assist to behaviourally buffer individuals against the vagaries (that is, unexpected and unpredictable changes) of the external environment. The evidence that enlarged brain provides a survival advantage when faced with novel challenges has been observed in birds [7]. For example, birds with large brains have been found to show higher survival in novel or altered environments, as well as in their native ranges attributable to their enhanced behavioural flexibility[8]. Intelligence is very difficult to measure in humans, let alone in animals. The encephalization quotient (EQ) proposed[9] has been employed as one of the measures of intelligence or the ability of an animal to cope with newly developed challenges and obstacles in its environment. EQ as defined[9] is the ratio between observed and expected brain size for a given body weight. The explanation to this was that in this system, a mammal with a brain/body size ratio of an EQ value equal to 1.0 is considered to have average EQ[1]. A value of EQ less than 1.0 may be associated with a less than average level of attributes that might be interpreted as “intelligence”; conversely, EQ value higher than 1.0 may be associated with more than average intelligence. Mammals with EQ values near and above two, e.g. primates and elephants, have been observed to make and use tools[10]. Tool usage among elephants includes the use of stick to scratch their backs and the use of twigs and branches under their back feet to prevent sinking in soft ground.Encephalization, or brain size larger than expected from body size, has long been considered to correlate with improved cognitive abilities across species and even intelligence[11]. The EQ was viewed as an ability of an animal to cope with newly developed challenges and obstacles in the environment[1]. How brain properties such as absolute and relative size and number of neurons relate to cognitive abilities has been a long-standing question in neuroscience. One of the widely used parameters is the EQ[9]. The EQ was first proposed as a useful parameter to correlate with intelligence based on the observation that some animals and humans seem to have larger brains than expected from their body size. In rodents, it was showed that direct relationship between EQ and somatic neuronal quotients (SNQ) indicates that more encephalised species have a higher number of neurones in excess for a given body size[11]. In the present study, relative brain and body weights of Jerison[9] were used and neurones were not counted, to warrant comparison with the later author[11]. Encephalization quotient values have been documented for man and a number of animals. For example, the elephant has an EQ of 1.12, horse, 0.9; the sheep, 0.8; the rat, 0.0.4; the mouse, 0.5, the rabbit, 0.4; the European cat, 1.14 and the ring-tailed lemur, 1.449. Others like the gorilla have an EQ range of 1.40 - 1.68; the chimpanzee, 2.18 - 2.45 and humans, 7.33 - 7.69[12]. Similar values for the grasscutter (GRC) and African giant rat (AGR) are lacking in the available literature. Body mass plays a central role in allometric study, because of all the body organs it contain and their physiological processes and brain is considered to be close to the body mass. It was therefore, the aim of the present study to provide for the first time the comparative brain-body size biometrical data for Africa’s biggest rodent after porcupine, the GRC[13-14], and largest muroid species, the AGR[15]; factors that place them among the preferred rodents of interest both in terms of scientific research and for their meat in tropical Africa. The study provides anatomical data that may be valuable in understanding the behavior of these rodents, as well as aid their domestication and production.
2. Materials and Methods
2.1. Sources of Animals and Study Location
- A total of twenty five rodents; made up of thirteen GRCs (six males and seven females) and twelve AGRs (six males and six females) were used in the study. The AGRs were live-trapped in the wild, in Zaria (11o10/N, 07o38/E), located in the Northern Guinea Savannah zone of Nigeria, and reared under laboratory conditions in the Department of Veterinary Physiology and Pharmacology, Ahmadu Bello University, Zaria. They were obtained in this department and transferred in laboratory cages to a nearby Veterinary Anatomy Research Laboratory, where the research was conducted.The GRCs were purchased from a breeder farm in Otukpo (07o13/N, 08o05/E), located in the Southern Guinea Savannah zone of Nigeria. The male GRCs used had brownish perineal staining, which was taken as an index of sexual maturity in males[16]. The GRCs were transported by road in wooden constructed laboratory cages which had two compartments (for males and females), measuring 50 cm (height) by 40cm (width) and 40cm (length), to the Anatomy Research Laboratory, Department of Veterinary Anatomy, Ahmadu Bello University, Zaria, Nigeria, where they were kept under room temperature for three days before the experiments began. The rodents were fed elephant grass (Pennisetum purpureum), supplemented with grower’s chick mash and given access to water and feed ad libitum.
2.2. Brain Extraction and Fixation
- The body weight of each rodent was obtained with a digital electronic balance Mettler balance P 1210, Mettler instruments AG. Switzerland) and the length using twine and meter rule. The rats were then euthanized by an overdose of chloroform anaesthesia (within 3-6 minutes) in a closed container. The head of each rodent was decapitated at the atlanto-axial joint using a small sharp knife and forceps. The head was weighed and its length measured before it was skinned and stripped of all muscles. The brain was then extracted from the skull as described[17], but with some modifications due to the peculiarity of the rodents. The brains were grossly examined for pathological lesions and were found to be apparently normal. The brains were weighed with a mettler balance, (Model P 1210, AG, Switzerland, with sensitivity of 0.01g) and their lengths obtained using a digital vernier caliper (MG6001DC, General Tools and Instruments Company, New York).
2.3. Statistical Analysis
- All Statistical analyses were performed using computerized statistical software (Graph Pad Prison, Version 3.10, 2009). The brain and body weights were expressed as mean and standard error of the mean (Mean ± SEM), and their differences between the male and female were analyzed using the unpaired t test, with Welch corrected. Pearson's Correlation Coefficient (r) was used to compare the relationships in weights and their P values were determined between sexes. Values of P ≤ 0.05 were considered significant.
2.4. Brain - Body Allometry Using Encephalization Quotient (EQ)
- The EQ was calculated from the data obtained on the brain and body mass of each rodent using the following equation [9]:
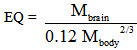 The interpretation of EQ was based on Shoshani et al.[1], where species with EQ values equal to 1.0 are considered to have average intelligence; values less than 1.0 are associated with less than average level of attributes that may be interpreted as ‘‘intelligence’’; and values higher than 1.0 are linked with more than average intelligence.
The interpretation of EQ was based on Shoshani et al.[1], where species with EQ values equal to 1.0 are considered to have average intelligence; values less than 1.0 are associated with less than average level of attributes that may be interpreted as ‘‘intelligence’’; and values higher than 1.0 are linked with more than average intelligence.3. Results
3.1. Morphometric of the Brain, Body and EQ
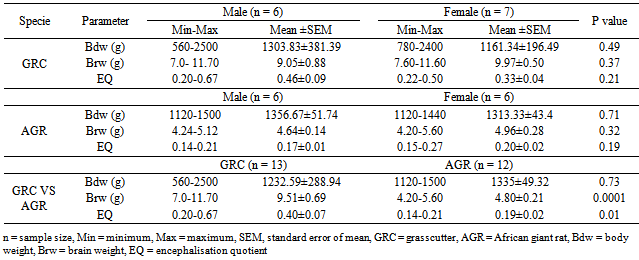
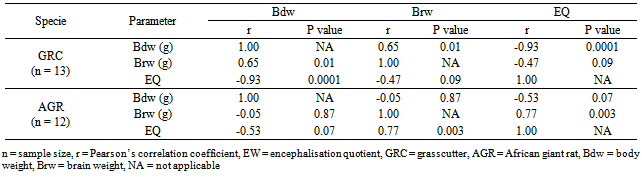
- Table 1 presents the body and brain weights and EQ data of the GRC and AGR. In the GRC, the mean body weights values was observed to be higher in the males (1303.83 ± 381.39 g) than in the females (1161.34 ± 196.49 g), though not statistically significant (P > 0.05). Also, in GRC, the brain weight in the female ranged from 7.60 – 11.60 g and had a mean value (9.97 ± 0.50 g) which was slightly higher than in the males (.97±0.50 g). As in the body weight, the male mean EQ (0.46 ± 0.09) was also higher than its female’s counterpart (0.33±0.04), but was not statistically significant (Table 1).For African giant rat, only the mean body weight (1356.67 ± 51.74 g) of the males was slightly higher than the female’s value (1313.33 ± 43.4 g). In the same AGR, the mean brain weight (4.64 ± 0.14 g) and EQ (0.17 ± 0.01) were lower than those in the female (4.96 ± 0.23 g and 0.17 ± 0.02), respectively, but were not statistically significant (Ps > 0.05) (Table 1).Computation based on species showed that the mean body weight of the AGR (1335.33 ± 49.32 g) was higher than that of the GRC (1232.59 ± 288.94 g). Contrariwise, there were extremely significant differences between the mean brain weight of GRC (9.51 ± 0.69 g) and the AGR (4.80 ± 0.21 g) (P < 0.0001). Also, the mean EQ in the GRC (0.40 ± 0.07) was significantly higher than in the AGR (0.19 ± 0.02) (P < 0.01) (Table 1). Table 2 presents the correlation matrix of relationships between the body, brain and EQ in the GRC and AGR. In the GRC, the body weight had a significant positive correlation brain weights (r = 0.65, P < 0.01) and extremely significant negative correlation with the EQ (r = -0.93, P < 0.0001). The negative correlation between the mean brain weight and the EQ was not quite significant (r = -0.47, P < 0.09).In the African giant rat, the correlation between the mean body weight and EQ was negatively and not quite significant (r = -0.53, P < 0.07). The mean brain weight had significantly positively correlated with the EQ (r = 0.77, P < 0.003) (Table 2).
|
|
4. Discussion
- GRCs of either sex attain higher adult body weights than AGRs[14-15], but some of the GRCs used in the present study weighed less than the AGRs probably because the later were older, having stayed longer in captivity, while the former were mainly young adults. Results of the study showed that the absolute mean brain weight/size of the GRCs was heavier/larger than that of the AGRs, even though the body mass of the former was less. In the GRC, brain weight correlated positively and significantly with body weight (r = 0.65, P < 0.01), whereas in the AGR the correlation was negative and non-significant. Positive correlation between the body and brain weights in the GRC shows that one could be used to predict the other, i.e. as one increases, the other also increases. Thus, body weight in the GRC may be used to predict brain weight, just as body measurements have been used to predict body weight in the GRC[14]. The finding agrees with the hypothesis which states that “brain size usually increases with increase in body size in animals” (positive correlation), that is, large animals usually have larger brain than smaller animals[18]. The non-significant correlation between brain and body weights of the AGR may be a result of the relatively little variation in the body weights of individual AGRs used for the study, compared to the wide variation observed in the body weights of the GRCs. The results show that AGRs of the same body weight had a predictable brain weight. Some authors considered factors like ecology, physiology and behavioural differences in animals[19-20] to affect neuroanatomical variables such as the brain and body weights. In another report, brain size and morphology is suggest to be related to information processing, storage and retrieval needs, just as gut size and morphology have been successfully related to the feeding habits of different animal species (21). It also been noted that differences in life-style, including dietary habits and patterns of development of the young were associated with variation in brain weight that is independent of the effect of body weight[21]. Knowledge of mammalian brain size is relevant to neuroscience because it reflect it macro and micro-morphology and their internal connectivity[20]. In rodents, brain size is related to factors like complex habitats, specialized diets and nocturnal behaviour[19]. Also, in squirrels, it has been reported that arboreal squirrels have relatively larger brain size than terrestrial species[22]. The rodents, GRCs and AGRs used in the present study have behavioural differences; like feeding habits, development and life-style, hence, variations in their brain size is expected. The variations in the brains of GRC and AGR obtained in the present study may be a useful tool to explain their behavioural differences in the wild, such as climbing, burrowing abilities and feeding. This complex behaviour of rodents in their ecosystems is said to be related to their intelligence[11, 23]. The relatively large brain size of the GRC, when compared with the AGR, could be related to their behavioural activity in their ecosystems. The relationship between brain structure and ecological variations, such as acquisition of food and locomotion in rodents has been documented[22]. Both the GRCs and AGRs are said to occur in close proximity to farmlands and human settlements, but the extent of their ecological distribution and dietary range differ[24-25]. AGRs are good climbers, using the long tail for balancing, but the GRCs with short tails have short range of climbing ability with little or no balancing support from its short tail (24). Using tail length as a climbing ability in Peromyscus, it was showed that brain size increased with tail length[26]. These authors therefore supported the hypothesis which states that relatively, large brains are required to navigate through structurally complicated habitats. The present results showed that the GRC with a relatively larger brain had a shorter tail than the AGR indicating that Isenberg and Wilson’s hypothesis may hold true within, but not across species of rodents.Brain size has also been correlated with life history. That brain weight varies very little during adult’s lifetime, but may be more correlated with some morphological life history or environmental variables than body weight[27]. In agreement with this report[27], other researchers reported that larger-brained species have longer gestation period, mature later and have increased lifespan[28]. The present finding that GRCs have relatively larger brains than AGRs corroborates the above reports because the former[29-30] have longer gestation period and lifespan than the latter[31].Another important factor in explaining the relationship between brain and body weight is the stage of maturation of different organs, including the brain at the time of birth. Altriacial mammals (such as humans, marsupials, cats, dogs and some rodents) and birds (such as woodpeckers, owls and passerines) have somatically immature brains at birth; hence they are incapable of moving around on their own soon after birth or hatching[12]. On the other hand, precocial mammals (such as ruminants and some rodents) and ground nesting birds (such as ducks or turkeys) have relatively large or mature brains at birth relative to their body size; hence their ability to fend for themselves[32]. As adults, however, altriacial animals end up with comparatively larger brains than their precocial counterparts do[12]. Evidence from literature indicates that GRCs are precocial[33], while AGRs are altriacial[31] rodents. One would, therefore, expect the adult GRC brain to be relatively smaller than that of the adult AGR at adult age, but it was not so. Rather, the adult GRCs brains were found to be relatively much larger than those of the AGR (P < 0.0001). The finding was not entirely surprising since other related biological rules have been found not to always hold true. In the present work, the calculated mean EQ values for GRC and AGR (0.40 ± 0.07 and 0.19 ± 0.02, respectively) with significant difference (P < 0.01) in favour of GRC, may suggest that these rodents have better memory storage and processing ability and, hence, are more intelligent than AGRs. Comparatively, both rodents are of less than an average (that is 1.0) intelligence[1]. The mean EQ of GRC was greater than those reported for the rat and rabbit, while the EQ of the AGR was smaller than the corresponding values reported for the above species[12]. The EQ value for male GRCs was slightly higher than it female counterpart, while it is slightly higher in the later than former in AGR, though without statistically significant (Ps > 0.05), indicating sexual dimorphism in the level of intelligence between species. The finding agrees with that of[11], who observed no sex difference in EQ values of six other rodent species, including the guinea pig, gouti, hamster and capybara. Also, it has been observed that variations in EQ values across these rodent species did not correlate significantly with parameters such as their absolute number of neurones or neuronal density[11]. The most intelligent mammals, the humans, have the largest absolute brain size, which forms 2% of the body mass, whereas shrews, the smallest mammals who exhibit supposedly much less cognitive and behavioural flexibility, have brains of up to 10% of their body mass[34]. Some authors therefore considered the relationship between brain and body weight as not being enough to determine intelligence[12]. Absolute brain size may, therefore, play a more important role in determining intelligence level. The results of the present study show that the GRCs had absolute brain weights that almost doubled those of the AGRs, which further imply a higher intelligence level for the former. The male GRCs mean EQ value was higher than that of the females even though the brain weight value was slightly lower than in the females. In the wild, it is of interest to note that GRCs are polygamists; one male to four or five females. They create trail through grass and reeds that give shelter and usually move in groups or colonies, composed of this male leading other females in their search for food and water sites[24]. This may suggests that males are likely to be more intelligent than the females. In AGR, the mean body size value was slightly larger than in males, but their brain and EQ values were higher in the females, though not statistically significant (P > 0.05). In the AGR, females had higher EQ values than male, which corresponded well with the report[1] which showed a higher mean EQ value for female’s elephant than their male’s counterpart. The reason may be that females reach puberty and physical maturity earlier than males, or due to specie variations. For example, in three species of Nigerian small ruminants, female Red Sokoto goats, West African Dwarf goats and West African Dwarf sheep, have heavier brain weights than their male counterparts, although all the values of their body weights were still higher than their male counterparts[35 - 37]. There existed a positive significant correlation between the body and brain weights (r = 0.65, P < 0.01) in the GRC and between the EQ and brain weight (r = 0.77, P < 0.003) in the AGR. It shows that one could be used to predict the other; as one parameter increases, the other also increases. This finding in the GRC agreed with the hypothesis that state that “brain size usually increases with increase in body size in animals”[18]. We failed to find a significant positive correlation between the body weight and EQ in both species, and between brain and body weights in the AGR, and have no good reason (s) to give on this respect. We therefore suggest that further studies with larger sample sizes, as well as covering different age groups.
5. Conclusions
- In conclusion, the brain weight could be used to estimate the body weight in the GRC and EQ in the AGR. The GRCs may possess higher intelligence than AGRs, although both rodents have less than average intelligence. Furthermore, there were sex differences in the level of intelligence; with GRCs males and AGR females slightly having higher EQ and their counterparts.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML