-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2013; 2(3): 39-49
doi:10.5923/j.neuroscience.20130203.02
Non-Pharmacological Interventions for Enhancing Brain Plasticity and Promoting Brain Recovery: A Review
Farheen Farzana1, Yog Raj Ahuja1, Vemula Sreekanth2
1Department of Genetics and Molecular Medicine, Vasavi Medical and Research Center, Hyderabad, 500004, India
2Department of Neurology, Apollo Hospital, Hyderabad, 500034, India
Correspondence to: Farheen Farzana, Department of Genetics and Molecular Medicine, Vasavi Medical and Research Center, Hyderabad, 500004, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Despite the incredible advancements made in the field of neuroscience, neurodegenerative disorders arising from the pathological forms of neural plasticity, continue to remain the primary cause of increased death rate. While significant progress has been made in the introduction of novel pharmacological approaches for treating neurodegenerative disorders, minimizing the unpleasant adverse effects associated with these medications, still remains a far-fetched dream. This has led to numerous pre-clinical and clinical studies that have accumulated considerable amount of evidence, supporting the effectiveness of non-pharmacological interventions for slowing the progression of a wide range of neuropsychiatric disorders. The primary objective of this review is to provide scientific evidence on the efficacy of non-pharmacological interventions like environment enrichment, cognitive stimulation therapy, physical exercise, social interactions, dietary modifications and relaxation techniques that can be in co-operated in your lifestyle for preventing and reversing age-associated cognitive decline. It emphasizes the importance of adapting a combinatorial approach and utilizing the potential of both, drug-based and non-drug based therapies for managing the symptoms of neurodegenerative disorders, since these conditions are caused by an interplay between genetic and environmental factors.
Keywords: Neurodegenerative disorders, Non-pharmacological interventions, Cognitive decline, Brain plasticity
Cite this paper: Farheen Farzana, Yog Raj Ahuja, Vemula Sreekanth, Non-Pharmacological Interventions for Enhancing Brain Plasticity and Promoting Brain Recovery: A Review, Research in Neuroscience , Vol. 2 No. 3, 2013, pp. 39-49. doi: 10.5923/j.neuroscience.20130203.02.
Article Outline
1. Introduction
- Over the last decade, the world has witnessed a rapid increase in the prevalence of neurodegenerative and psychiatric disorders[1], owing to the lack of access to drugs that target the underlying cause of the disease and cost-effective treatments for curing these disorders. Even though several drugs are available in the market for treating the symptoms of these disorders, the number of unpleasant and long term side-effects like cardiovascular and metabolic effects associated with these medications are phenomenal [2,3]. Furthermore, among the large proportion of patients who receive pharmacological treatments, only a small portion of the population experiences full remission while the others do not respond in the same manner. Hence, in order to overcome treatment resistance and the troublesome side-effects associated with these medications, introduction of non-drug based approaches, alone or in combination with drug-based therapies will serve as an effective strategy to guide and accelerate the natural process of brain recovery. Even though, a number of randomized controlled trials have recorded the positive effects of a growing number of non-pharmacologic interventions that can promote nerve regeneration, they have received far less attention and funding than drug-based research[4]. Hence, the aim of this review article is to highlight the different non-drug based therapies including lifestyle changes that can enhance brain plasticity and improve the quality of life of patients suffering from age-associated neurological disorders.
2. Can Neural Plasticity be Enhanced in the Adult Brain?
- Brain deterioration and a subsequent decline in cognitive and motor functions are considered to be common characteristics that accompany the normal process of ageing. However, contrary to the early dogma that the brain is fully formed during childhood and neurons once dead could never be substituted by new ones during adulthood, it is now known that the adult brain retains some plasticity, albeit to a lesser extent than the younger population and continues to modify its structural and functional organization and function, in response to an environmental stimulus[5]. Brain plasticity or neural plasticity is exhibited in two forms, namely synaptic plasticity, which refers to the strengthening and weakening of synapses that leads to learning and memory formation[6] and adult neurogenesis, a process by which new neurons are generated from neuronal progenitors to compensate for the lost or damaged neurons[7] (As shown in Figure 1).
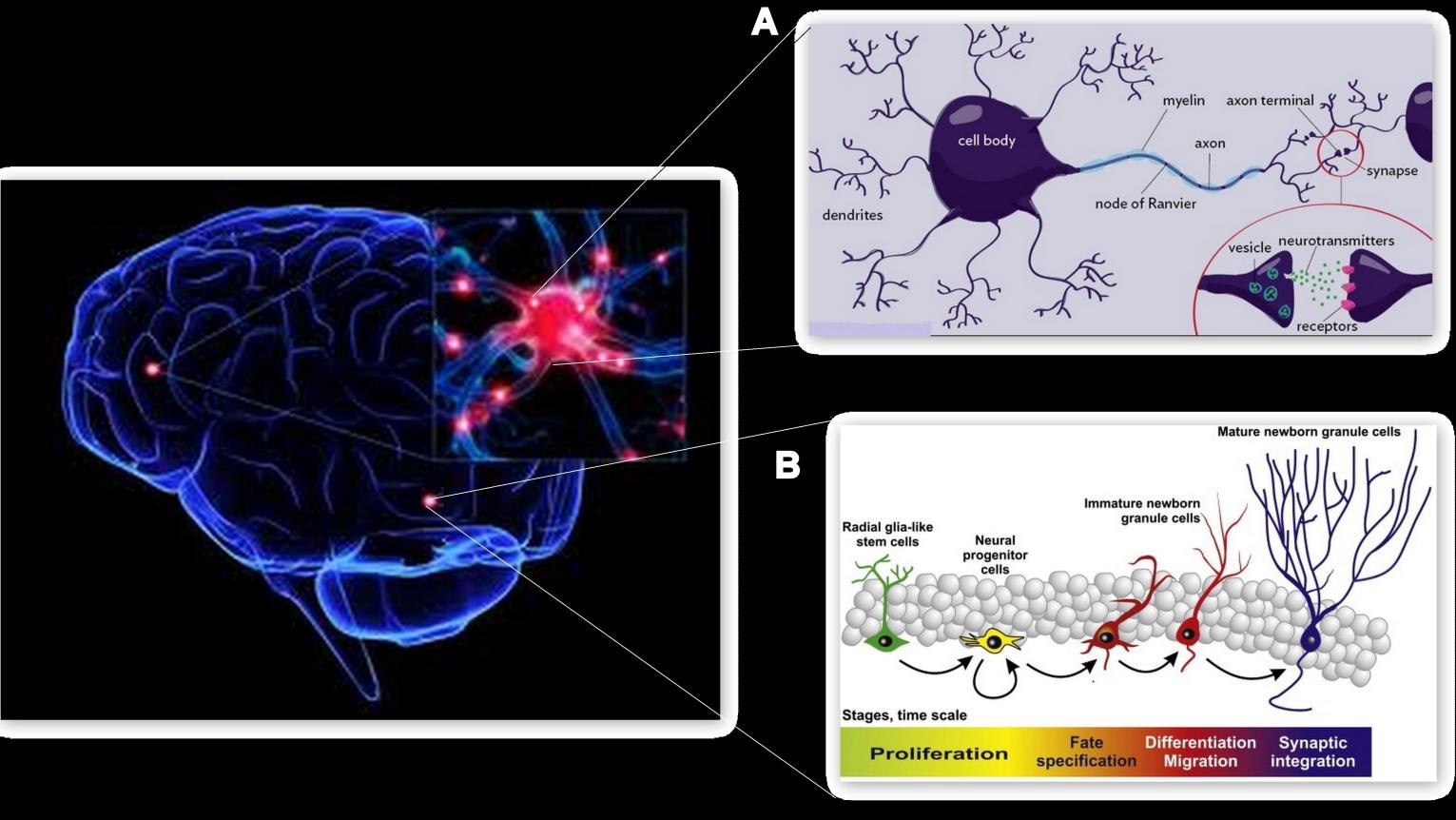 | Figure 1. Structural elements of brain plasticity. A, Synaptogenesis: Formation of synapses between two neurons. B, Neurogenesis: Generation of new neurons from neuronal stem cells |
- Decades of research have demonstrated the efficacy of brain-plasticity based approaches for slowing down the progression of a wide range of brain disorders in humans[8]. However, given the number of challenges that the brain faces as a part of normal ageing, maintaining brain plasticity in adulthood, continues to remain a major challenge faced by today’s world. Nevertheless, a recent innovative study conducted to examine the influence of age on the abolishment of brain plasticity revealed that the loss in number of neurons with age is compensated for, by an increase in dendritic branching, an essential component required for enhancing brain plasticity[9]. This has led to a considerable number of studies that have gathered substantial amount of evidence supporting the efficacy of non-drug based therapies for reversing brain decay, by utilizing the brain’s natural capacity for plasticity that is retained even during adulthood and old age[10].Among the various molecular mediators that augment the process of neurogenesis in the adult brain, nerve growth regulators or neurotrophins especially, Brain-derived neurotrophic factor (BDNF) has been established as a candidate target molecule for enhancing neural plasticity[11]. Neurotrophins play an important role in the epigenetic regulation of neural plasticity by promoting the proliferation and differentiation of neural precursor cells into new neurons[12] and enhancing synaptic transmission, during brain development and at adulthood[13]. Many scientific studies have established a strong correlation between the dis-regulation of BDNF levels, impaired neurogenesis and an increased susceptibility to several neurological and psychiatric disorders[14] like Dementia[15], Alzheimer’s disease[16], Parkinson’s disease[17], Schizophrenia[18], Huntington’s disease[19], Autism[20] and Depression[21].
3. Non-Pharmacological Approaches for Enhancing Brain Plasticity
- Over the past few decades, several lines of scientific evidence and clinical literature have established the beneficial effects of non-pharmacological therapies in enhancing the regenerative power of the brain, thereby promoting additional clinical research in this area[22-24]. However, lack of awareness in terms of the application of non-pharmacological therapies has limited their usage and hence, a review of the available scientific evidence supporting their efficacy and safety is important to place practice recommendations on a sound evidence-base. In the past few decades, research on brain plasticity and its induction by experience-related changes has rapidly gained momentum and attracted a great deal of public interest. Even though non-pharmacological interventions have been criticized for lack of vigor, there is growing evidence supporting the beneficial effects of these interventions for enhancing brain plasticity[25]. In this review, we summarize research supporting the benefits of non-pharmacological interventions for enhancing neural plasticity in the adult mammalian brain and reversing age-associated cognitive decline.
3.1. Environmental Enrichment
- Environment enrichment takes into the account the crucial role played by the complex interaction between genetic factors and environmental modifiers in the etiology and progression of brain and psychiatric disorders[26]. Using a range of rodent models, environmental enrichment is often provided in the form of improvised housing conditions that enable an active lifestyle and a greater level of social, cognitive and sensorimotor stimulation, in order to promote neuro-rehabilitaion after brain damage[27]. Enriched cages are engineered to be larger than the standard cages to accommodate more animals for promoting social interaction and consist of running wheels for increasing physical exercise. In addition, in order to stimulate cognitive and exploratory behavior in rodents, these cages consist of diverse equipment of various sizes, shapes, color and composition like ladders, nesting material, cardboard boxes, numerous polycarbonate and plastic play tubes that are changed on a regular basis to provide novelty[28]. Several lines of evidence have established the role of stimulating environment in inducing significant synaptic changes[29] and morphological alterations in the dendate gyrus of the hippocampus[30], which manifest in the form of better performances in memory[31], learning and spatial navigational tasks in rodents[32]. Exposure of mice to an enriched environment have been shown to delay the progression of disease in genetic models of many neurodegenerative disorders like Alzheimer’s disease[33], Parkinson’s disease[34] and Huntington’s disease[35]. Moreover, environment enrichment has also been shown to improve cognitive functioning in transgenic mice over-expressing amyloid precursor protein and/or presenilin-1, thereby demonstrating the importance of education and cognitively demanding jobs on reducing the risk of dementia[36].The positive aspects of environment enrichment, especially wheel running and learning have been strongly correlated with enhanced neural plasticity[37] and an increased rate of hippocampal neurogenesis, as shown by an increase in the number of new neurons in the hippocampal region of mice[38]. This increase in the rate of neurogenesis in enriched mice has been shown to be accompanied by a significant increase in the expression levels of Brain-derived neurotrophic factor, a member of the nerve growth family that is actively involved in enhancing neuronal plasticity in the adult brain[39]. In addition, environment enrichment promotes synaptic transmission by up-regulating the expression of genes involved in postsynaptic signal transduction and down-regulating the expression of genes associated with the reuptake of neurotransmitters at the presynaptic junction, thereby improving synaptic plasticity [40]. Even though adult hippocampal neurogenesis normally declines with age, adult mice exposed to enriched environment showed reduced levels of lipofuscin in the dentate gyrus, decreased age-dependent degeneration and a fivefold increase in adult hippocampal neurogenesis, when compared to mice housed in standard cages, indicating the sustained effect of environment enrichment on brain plasticity[41]. In humans, cognitive enrichment in the form of educational attainment and occupational status has been shown to induce neuroplasticity that not only strengthens the existing neural networks, but also recruits alternative neural networks to permit normal cognitive functioning in an injured brain[42]. Therefore, numerous studies have investigated the potential of psychological interventions, physical therapy at social interaction at providing an enriched environment and improving cognition and quality of life of patients suffering from brain disorders.
3.2. Cognitive Stimulation Therapy
- Cognitive stimulation therapy (CST) aims at improving various aspects of neuropsychiatric symptoms such as mild to moderate cognitive impairment, impaired social interaction and communication skills and a poor quality of life in the elderly population suffering from dementia[43]. It is derived from Reality orientation therapy, a psychological intervention used in geriatric health care to improve cognition in the confused and disoriented elderly population suffering from dementia[44]. CST is a structured and cost-effective program[45] that is offered either on an individual basis[46] or in small groups of 4-5 people[47]. Based on the principle that lack of cognitive activity speeds up the process of cognitive decline, CST provides intensive training using a range of intellectually stimulating activities like puzzles, word games, team games, memory games, shape and color recognition, pattern recognition tasks, mazes, complex video games, group discussions and indoor activities like knitting, gardening and baking that can keep patients cognitively active, improve concentration and promote thinking and memory[48]. A study involving clinical evaluations of 700 elderly population, conducted over a period of five years revealed that cognitively inactive people are 2.6 times more likely to develop Alzheimer’s disease than cognitively active people, thereby establishing frequent cognitive activity during old age a means of reducing the risk of developing Alzheimer’s disease[49]. Besides establishing the role of CST in improving working memory and quality of life in Alzheimer’s patients, when conducted alone[50], researchers have also shown the additional benefits of CST when used in combination with the administration of acetylcholinesterase inhibitors for reversing the process of verbal and functional decline and decreasing negative emotional symptoms, thereby improving overall global performance in Alzheimer’s patients[51]. These studies support the hypothesis that there is considerable brain reserve and potential to improve cognitive functions in older adults and hence, cognitive stimulation therapy has gained wide acceptance for delaying cognitive decline associated with dementia.
3.3. Physical Exercise
- Extensive research has been conducted on the profound effect that physical activity has on promoting brain health and plasticity[52] and its efficacy in preventing age-associated cognitive decline and neurodegenerative disorders[53]. A substantial amount of evidence has shown the impact of both, voluntary exercise in the form of wheel-running[54] and forced exercise in the form of regular treadmill running[55] on the rate of hippocampal neurogenesis and a subsequent improvement in performance in learning and memory tasks in adult mice. In addition to improving cognition and motor abilities in healthy older adults, physical activity has also been found to have a neuroprotective effect on cognitive functions[56], balance, strength and mobility[57] in elderly individuals suffering from dementia and related cognitive impairments. However, these structural changes and an enhancement in synaptic plasticity were observed only in rats subjected to 56 days of long-term wheel-running, as opposed to 14 days of short-term wheel-running, which emphasizes the need for long-term periods of physical exercise to facilitate its structural and functional benefits on the brain[58]. Also, a study examining the effect of intensity of physical activity required to normalize corticomotor excitability and increase gait speed, stride length and step length in patients with early Parkinsons disease has demonstrated greater benefits using high-intensity exercise than low- and zero-intensity exercise[59]. Furthermore, a study conducted on older adults by Kramer et. al[60] has found that a six month intervention of moderate aerobic exercise in the form of walking as opposed to stretching and toning exercises, can dramatically enhance executive functions in the brain, thereby re-enforcing the view that plasticity or the potential for positive change is maintained even during adulthood. While the molecular mechanisms by which exercise enhances brain plasticity have not been completely elucidated, evidence from numerous studies have established Brain-derived neurotrophic factor (BDNF) signaling as the candidate neural mechanism for facilitating exercise-dependant modulation of learning and memory and enhancing brain plasticity[61-63]. However, a meta-analytic study conducted by Colcombe and Krame[64] have reported that the effect of aerobic exercise on increasing BDNF levels and cognition have been found to be more pronounce in studies that included more women than in studies that included fewer women. This has been attributed to be the interaction between estrogen and BDNF, as supported by a study that showed no impact of aerobic exercise on the up-regulation of hippocampal BDNF levels in mice that were deprived of estrogen[65]. In addition, the same study showed that an increase in BDNF levels due to physical activity was found to be more pronounced in mice that underwent estrogen replacement therapy in combination with exercise than mice subjected to estrogen replacement therapy alone. This emphasizes the importance of physical exercise in post-menopausal women, especially in women undergoing hormone replacement therapy and may explain the benefits of estrogen replacement therapy in increasing the positive effects of exercise and mitigating the rate of brain atrophy in late adulthood. Moreover, physical exercise has also been shown to augment the effect of hormone therapy and offset the negative effects of prolonged hormone treatment replacement like memory impairment and irreversible neuronal damage[66]. Besides increasing BDNF levels, physical exercise has also been found to influence the gene expression profile of various other mediators of neuroplasticity in the adult brain such as Growth hormone (GH)[67], insulin-like growth factor I (IGF-I)[68], synapsin I[69] and Fibroblast growth factor-2 (FGF-2)[70] that aid in initiating molecular cascades that are crucial for the onset of neurogenesis in the adult brain. At the supramolecular level, physical exercise has been found to be effective in increasing dendritic length, spine density and neurogenic activity in dentate gyrus of the adult brain[71]. Evidence from numerous scientific studies have confirmed the role of physical activity in promoting brain vascularization[72], neuronal repair following brain injury[73] as well as reducing the risk of memory impairment and dementia in late adulthood by selectively reversing the loss of hippocampal volume with age[74]. Stress and elevated levels of glucocorticoids are known to inhibit neuronal growth and hippocampal neurogenesis in the adult brain[75]. There is presently a growing set of evidence that supports the positive effects of physical exercise in counteracting the effect of stress by reducing the levels of stress hormones[76] and increasing the availability of BDNF in the hippocampus, an important mediator of neurogenesis and plasticity in the adult brain[77]. On the other hand, a sedentary lifestyle in urban areas[78] and physical frailty[79] have been associated with an increased rate of cognitive decline and a higher risk of cognitive impairment in older adults. Hence, physical activity can act as a powerful effector of brain pathology by reversing stress-induced impairment of adult neurogenesis and increasing resistance to brain injury.
3.4. Social Interaction
- Social interaction forms an integral part of our lives and is one of the most effective life style modifications that can bring about significant improvements in our cognitive abilities. In fact, a study has revealed that engaging in a 10 minute session of social interaction involving mentally stimulating conversations can enhance brain plasticity by improving cognitive abilities like learning, memory, attention and control[80]. Contrary to social interaction, isolation has deleterious effects. Oligodendrocytes are cells that actively participate in the formation of myelin sheaths around the neurons and promote nerve transmission[81]. Socially isolated rats showed a remarkable decrease in the density of oligodendrocytes in the prefrontal cortex, a region in the brain that governs emotional and cognitive behavior. Rats are, in general, highly motivated to be social; however when exposed to a novel rat after 8 weeks of isolation, they did not show any interest in interacting with the new rat, thus turning into a model of social avoidance and withdrawal[82]. Hence, social isolation can disrupt nerve transmission and affect brain plasticity to a great extent, thereby leading to many demyelinating and psychiatric disorders. Moreover, it was found that the effect of social interaction on promoting brain recovery in mice, following ischemia, was superior to wheel-running and an enriched environment that comprised of free physical activity[83]. In addition, social isolation has been linked to increased susceptibility to stress, which in turn, attenuates the process of neurogenesis reduces the expression levels of brain plasticity markers in the hippocampus region and pre-frontal cortex[84]. In today’s world, owing to fewer social connections, decline in the number of organizations, family dinners and gatherings that involve one-on-one conversations, people have been finding it hard to establish a close relationship with someone to share their innermost feelings and thoughts. This has led to a gradual decline in cognitive abilities, thus reducing the sense of well-being and making socially inactive people, more prone to mental illnesses[85]. A study conducted by researchers at the Rush Alzheimer's Disease Center has established emotional isolation or loneliness as a potent risk factor for Alzheimers disease. Hence, people who stay persistently lonely become more susceptible to the devastating symptoms associated with age-related neuropathology[86]. Humans are biologically wired to be social creatures and therefore, need to stay socially active by maintaining close friendships and healthy relationships in order to improve brain health.
3.5. Dietary Modifications
- Many years of research have demonstrated the beneficial effects of dietary modifications that incorporate calorie restriction, on reversing age-associated cognitive decline in both wild-type[87] and experimental models of Alzheimer’s disease[88], Parkinson’s disease[89] and Stroke[90]. Intake of proper diet leads to a reduction in oxidative stress that is primarily responsible for senescence-related loss of brain functions in neurodegenerative disorders[91]. At the molecular level, dietary restriction have been found to exert a neuroprotective effect by altering synaptic homeostasis[92], signalling cascades and long-term brain plasticity[93], thereby increasing resistance to withstand oxidative and metabolic insults.Most importantly, dietary restriction has been found to alter the expression of neurotrophins like Brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), which play a major role in increasing the rate of adult neurogenesis and enhancing brain plasticity, thereby slowing down the progression of neurodegeneration and increasing resistance to brain injury[94]. This has been supported by another study conducted in mice that has established the role of a diet rich in high saturated fat and refined sugar, a typical diet of many urbanized countries, in lowering hippocampal levels of Brain-derived neurotrophic factor (BDNF) and its downstream effectors like synapsin I, cyclic AMP-response element-binding protein (CREB) and growth-associated protein 43; factors that are crucial for maintaining neuronal growth, neurotransmitter release, and synaptic plasticity[95].Hence, calorie restriction, in conjunction with dietary modification, involving the incorporation of fruits, vegetables and foods that are enriched with nutrients that influence brain health and cognition[96], constitute an effective strategy for both preventing and reversing the progression of neurological and psychiatric disorders.
3.5.1. Diet Rich in Omega-3 Fatty Acids
- Long-chain Omega-3 polyunsaturated fatty acids namely docosahexaenoic acid and eicosapentaentoic acid form an important constituent of neuronal membranes and play a crucial role in maintaining cognitive abilities in late adulthood[97]. Decreased levels of Omega-3 fatty acids have been strongly linked with an increased rate of cognitive impairment, dementia and Alzheimer’s disease[98] and other neuropsychiatric disorders like schizophrenia, depression and attention deficit hyperactivity disorder (ADHD) [99,100]. A study conducted by Wu A et.al[101] has shown that a diet rich in Omega-3 fatty acids fed to mice, for a period of 4 weeks, not only improved the speed of nerve impulse transmission, but also protected neurons from oxidative damage induced by mild fluid percussion injury (FPI). The neuroprotective effect conferred by Omega-3 fatty acids against reduced plasticity and impaired learning and memory was found to be a result of the normalization of the levels of BDNF and its downstream effectors namely, synapsin I, and cAMP responsive element-binding protein (CREB).Hence, supplementation of Omega-3 fatty acids through a diet rich in fish, fish oils, sardines, walnuts, flax seeds, tofu, soybeans, olive oil, canola oil has been implicated to improve learning and memory and delay age-associated cognitive decline.
3.5.2. Diet Rich in Iron
- Given the vital role played by iron in brain oxygen transport, myelin production, morphology of neural networks and synthesis of neurotransmitters, iron deficiency in early stages of life can lead to irreversible alterations in brain development and neuronal functioning[102]. While iron overload also has been strongly associated with the pathophysiology of many neurodegenerative disorders[103], early-life iron deficiency has been shown to alter hippocampal volume by impairing hippocampal neurogenesis and reducing the expression levels of Brain-derived neurotrophic factor[104].During normal aging and age-related neurodegenerative diseases like Alzheimer’s and Parkinson’s disease, dyshomeostasis of iron has been commonly observed[105]. Hence, intake of foods rich in iron like dark leafy greens, beans, lentils, chickpeas, tofu, red meat, chicken, turkey, egg yolk, liver, oysters, dates, nuts and raisins can maintain optimum levels of iron that in turn, up-regulates the levels of BDNF and slows down the rate of age-associated neurodegeneration in the brain.
3.5.3. Diet Rich in Zinc
- Zinc has been found to play a critical role in regulating communication between nerve cells in the hippocampus, thereby maintaining cognitive stability and improving learning and memory[106]. Highly concentrated in the cortex, hippocampus and amygdala, zinc acts as a cofactor for many enzymes and zinc metalloproteins that are essential for synaptic neurotransmission and cellular signalling cascades[107]. Alterations of zinc dyshomeostasis both in the form of zinc accumulation and zinc deficiency have been considered an important factor for the onset of Alzheimer’s disease, depression other age-related neuropsychiatric disorders[108]. Indeed, zinc deficiency has been strongly linked with reduced hippocampal neurogenesis and increased neuronal death, thus leading to the impairment of synaptic plasticity and learning and memory deficits[109]. Also, a growing number of studies conducted in humans and rodents support the involvement of zinc deficiency in the pathogenesis of depression[110]. While zinc deficiency has been found to be a potent risk factor for treatment resistance to anti-depressants[111], zinc treatment in mice has been shown to mimick the activity of anti-depressants by increasing the expression of BDNF[112]. Hence, ensuring adequate intake of foods rich in zinc like nuts, seeds, liver, red meat, shellfish, oats and green peas can enhance brain plasticity and can have a great impact on brain health.
3.5.4. Diet Rich in Vitamin E
- Owing to its potent anti-oxidant activity, Vitamin E aids in scavenging toxic free radicals, thus counteracting the effect of oxidative damage on neuronal cells[113]. Elevated serum levels of two natural forms of vitamin E, namely tocopherols and tocotrienols, have been associated with a reduced risk of cognitive impairment in older adults[114]. Palm oil-derived alpha-tocotrienol, a natural Vitamin E molecule has been found to have the potential of attenuating the process of oxidative metabolism of arachidonic acid, a common mechanism that leads to the onset of several neurodegenerative disorders[115]. The anti-oxidant property of vitamin E has been linked to its ability of normalizing the levels of BDNF, and its downstream effectors like synapsin I and cyclic AMP-response element-binding protein (CREB) that are important modulators of neuronal and behavioural plasticity[116].Therefore, frequent consumption of foods rich in Vitamin E like tofu, spinach, avocados, broccoli, red bell peppers, tropical fruits, nuts, seeds, fish, olive oil and palm oil can enhance neuroprotection and delay the development of neurodegenerative disorders like Alzheimer’s disease[117].
4. Relaxation Therapies
- There is considerable epidemiological evidence supporting the association between chronic stress and impaired neuroplasticity[118]. Brain-derived neurotrophic factor (BDNF) and Neurotrophin-3, the two neurotrophic factors that play an important role in enhancing brain plasticity and preventing neuronal death, have been found to be lowered in rats that were exposed to chronic stress[119]. This has been supported by data obtained from human and animal studies that suggest an inverse relationship between stress hormones and BDNF levels[120]. Hence, abnormally high levels of stress hormones and a simultaneous reduction in the expression of BDNF plays an important role in stress-mediated changes in neuroplasticity and cognition [121].In order to relieve stress, several researcher have shown the benefits of various relaxation techniques like deep breathing meditation, yoga, Tai chi, aromatherapy and music therapy for ameliorating cognitive impairment associated with increases stress. Tai chi, a non-aerobic exercise originally developed as a means of self-defence, has now evolved as a powerful technique for promoting psychological well-being by reducing stress, anxiety and depression[122]. In fact, a study conducted by James A Mortimer et. al showed an increase in the brain volume and cognitive improvement in healthy individuals practicing Tai chi[123]. Meditation has been shown to have a neuroprotective effect through its ability to strengthen neuronal circuits, delay the process of neurodegeneration and reduce the risk of age-associated cognitive decline[124]. Indeed, yoga meditation have been associated with neuroplastic changes in the brain, as evident by an increase in grey matter volume[125] and elevated serum BDNF levels in depressed individuals[126].There is also a growing body of evidence on the beneficial effects of aromatherapy in reducing stress levels, as shown by a studies demonstrating the effect of lavender oil on reducing stress levels and pain intensity[127]; essential oils of salvia species on improving the quality of memory and mood[128] and aromas of peppermint showing a significant improvement in cognitive performance and mood levels in healthy individuals[129]. In addition, an interesting study conducted using a rat model of Alzheimer’s disease have shown the positive effects of the essential oils of Chamaecyparis obtuse in reversing neuronal apoptosis and memory deficits[130]. Hence relaxation techniques incorporated in one’s lifestyle can not only prevent the onset of neurodegenerative disorders, but can also suppress the progression of the disease by promoting adult neurogenesis during pathological conditions.
5. Conclusions
- Given the available evidence, we conclude that non-pharmacological interventions show promising results in reducing age-associated cognitive decline and offer hope to target the underlying cause of brain disorders by augmenting neurogenesis and preventing neuronal death, thereby stimulating neuronal regeneration in the adult brain. Besides promoting neuro-rehabilitation in pathological conditions, these therapies can also be adapted by the younger generation as preventive measures to delay the onset of neurodegenerative disorders in adulthood. Considering the fact that brain plasticity is maintained well into adulthood and old age, when devising strategies to combat age-related neurological disorders, it is best to adapt a combinatorial approach involving drug-based therapies, psychological interventions and lifestyle modifications to overcome the adverse effects of medications and facilitate plastic changes in the brain to speed up the process of recovery.
6. Future Research
- Even though the effectiveness of non-pharmacological interventions in enhancing brain plasticity has been well established, there are many unanswered questions with regard to the implementation of these therapies in real-life settings. Furthermore, there is a clear need for more randomized controlled trials and funding to systematically address the long term benefits of non-pharmacological intervention for enhancing brain plasticity. Also, a common or different molecular mechanisms underlying the beneficial effects of non-pharmacological interventions in enhancing brain plasticity still remain to be satisfactorily elucidated. Therefore, further investigations linking the benefits of non-pharmacological interventions for improving cognition, mood, quality of life, behavior and day-to-day functioning, with the measurement of the changes in the levels of molecular mediators of adult neurogenesis, can strengthen the existing evidence and support the theoretical basis of these non-drug based therapies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML