-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2013; 2(3): 31-38
doi:10.5923/j.neuroscience.20130203.01
Effects of Artemether and Artesunate on Social Behaviour and Pain Perception
Koofreh G. Davies1, Nnocent Edagha1, Ekpe Aribo2, Atim B. Antai2, Eme Osim2
1Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria
2Department of Physiology, Faculty of Basic Medical Sciences, University of Calabar, Calabar, Nigeria
Correspondence to: Koofreh G. Davies, Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study evaluated the effects of artemether and artesunate on social behaviour and pain perception using nesting score and animal models of pain namely, tail-flick test, hot plate test and formalin induced neurogenic response. Fifty mice were randomly divided into 5 groups. The control group received water only while the 4 experimental groups were treated respectively with 30mg/kg of artesunate, 60mg/kg of artesunate, 30mg/kg of artemether and 60mg/kg of artemether orally. Drugs were administered daily for 28 days. Social behaviour was not affected in this study. Artemether at high doses caused increase in pain parameters in hot plate test, tail flick and phase 1 of formalin test.A similar trend was produced by artesunate especially at 60mg/kg but the effect was not as prominent as that of artemether.In phase 11 formalin test, pain parameters were enhanced by artesunate at 30mg/kg and artemether at 60mg/kg. In conclusion, oral artemether and oral artesunate (to a lesser extent) at the dose of 60mg/kg caused suppression of acute pain. However, both drugs appeared to enhance slow pain.
Keywords: Artesunate, Artemether, Social Behavior, Pain Perception, Behavioural Parameters
Cite this paper: Koofreh G. Davies, Nnocent Edagha, Ekpe Aribo, Atim B. Antai, Eme Osim, Effects of Artemether and Artesunate on Social Behaviour and Pain Perception, Research in Neuroscience , Vol. 2 No. 3, 2013, pp. 31-38. doi: 10.5923/j.neuroscience.20130203.01.
Article Outline
1. Introduction
- Artemisinin, also known as Qinghaosu (Chinese: 青蒿素), and its derivatives are a group of drugs that possess the most rapid action of all current drugs against Plasmodium falciparum malaria[1]. The starting compound artemisinin, is isolated from the plant Artemisia annua, an herb described in Chinese traditional medicine. Chemically, artemisinin is a sesquiterpene lactone containing an unusual peroxide bridge. The endoperoxide bridge in the trioxanepharmacophere of artemisinins is believed to be essential for their anti-malarial activity, as replacement of one peroxide oxygen, with a carbon (e.g. in carbon -10-deoxyartemisinin) results in a derivative devoid of antimalarial activity[2][3]. Few other natural compounds with such a peroxide bridge are known [4]. Artemsinin was discovered in 1971 by agroup Chinese scientists following increasing parasite resistance to Chloroquine and today artemisinin-based combination chemotherapy (ACT) has become the first line treatment worldwide for P. falciparum malaria. Also, cell lines studies and some clinical trials have “unveiled” artemisinins aspotential anti-cancer drugs. Chemical modifications of artemisinin (reduction plus esterification) have enabled more potent and more soluble derivatives to be obtained, with improve bioavailability[5][6]. The most commonly used derivatives are artemether, methyl ether of dihydroartemisinin and artesunate, a hemisuccinate derivative of dihydroartemisinin[7].Although Artemisinins have been widely reported to be safe clinically[8], some studies (animal studies) have shown that these drugs are neurotoxic[9][12]. In all mammal species tested to date, these compounds have produced an unusual selective pattern of damage to certain brain stem nuclei i.e. precerebellar nuclei of the medulla oblongata and particularly those involved in auditory processing and vestibular functions i.e. the trapezoid nucleus, the gigantocellular reticular nucleus and the inferior cerebellar peduncle[10]. In rat, the target brainstem nucleusconsistently and most severely affected is the nucleus of the trapezoid body[11][12][13]. Changes in the affected neurons were loss of Nissl substance, perikaryonal swelling, margination of the nucleus (nucleus accentricity), nucleolar changes, and increased perikaryonal eosinophilia with occasional clumping of eosinophilic debris[10]. Neurotoxicity is commonly seen with parenteral route and prolonged administration. Oil soluble derivatives of Artemisimins are reported to be more toxic than the water soluble forms with brain stem nuclei being the most susceptible of the neuronal cells. Since brainstem is a major relay centre, it is conceivable that functional impairment following its damage may not be limited to only brainstem controlled functions. Despite the flurry of work done on artemisinins, nothing has been reported on its possible effect on behaviour. Since damage to the brainstem could affect behavior, our study sought to determine the effects of administration of high and prolonged doses of artemether and artesunate on some neurobehavioural parameters such as locomotion, exploration, anxiety/fear, learning/memory pain and social behavior, since there was little or no literature on those parameters before. We have already published findings on all except pain and social behaviour.The aim of this study therefore wasto investigate the effects of artemisinins on pain and social behavior of mice.
2. Materials and Methods
2.1. Animal Care
- Adult albino mice, fifty in number were housed singly in metabolic cages under standard laboratory conditions and were fed with pellet feed (Vital feed and flour mill limited, Edo, Nigeria). All animals were housed in a cross ventilated room (temp 22±2.5; humidity 65±5% and 12h light/12h dark cycle). A period of one week was allowed foracclimatization.
2.2. Drug Preparation
- Oral Artesunate marketed under the brand name “ARTESUNATR” manufactured by Neros Pharmaceutical limited, Lagos, Nigeria was purchased from a reputable pharmacy in Uyo, AkwaIbom State, Nigeria. The drug was reconstituted by adding distil water to produce the desired stock solution. Each freshly reconstituted suspension was used for a day.Oral Artemether suspension marketed under the brand name “Gvither” manufactured by Bliss GVS Pharmaceutical limited of India was purchased from a reputable pharmacy in Uyo, AkwaIbom State, Nigeria. The suspension was reconstituted by adding 100ml of water to each bottle (which contained 300mg of Artemether). The bottle was then tightly closed, inverted and shaken until all the powder was dispersed. This yielded a stock solution of 3mg of artemether per ml. Each reconstituted suspension was stored in a cool dry place at room temperature (25℃) and used within 7 days.
2.3. Animal Treatment
- Fifty (50) Albino Mice were randomly separated into 5 groups. Group 1 served as control and received only feed and water. Groups 2, 3,4 and 5 respectively received artesunate 30mg/kg (low dose), artesunate 60mg/kg (high dose), artemether 30mg/kg (low dose)and artemether 60mg/kg (high dose) daily for 28 days by gavage.
2.4. Experimental Procedures
- Pain was assessed by formalin test, hotplate test and tail flick test while social behaviour was assessed by nesting score.
2.4.1. Formalin Test
- In this test, 0.1ml of 10% formalin solution was injected into the plantar surface of the right hind paw of animals using a 29G needle, and the animal was placed immediately in the box. Pain-like behaviourslike licking/biting were recorded in phase I (0-5 minute after injection) and phase II response (20 minute after injection).Also, flinching and raising up of the paw were recorded as paw attention.
2.4.2. Hot Plate Test
- After setting the analgesiometer to the temperature of 50ºC, a beaker of 1litre capacity was placed and allowed to equilibrate with the temperature. The animals were then dropped in the beaker and the latency time to the first hind paw or/and jumping responses were measured (Eddy and Leimbach, 1953).
2.4.3. Tail Flick Test
- The tailof the mice was dipped in hot water maintained at 50ºC and the time taken before the first tail flick was recorded.
2.4.4. Nesting Test
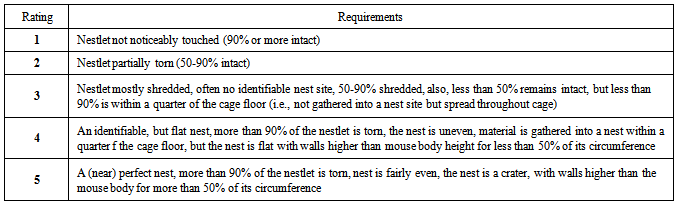
- A measured quantity of cotton wool was placed in the animal cages and the score was done 24 hours after (see table below).
|
3. Statistical Analysis
- Data collected during the study were expressed as mean ± standard error of mean (SEM), analysis of variance (ANOVA) and the student t-test was used for analysis. Values of P<0.05 were regarded as significant.
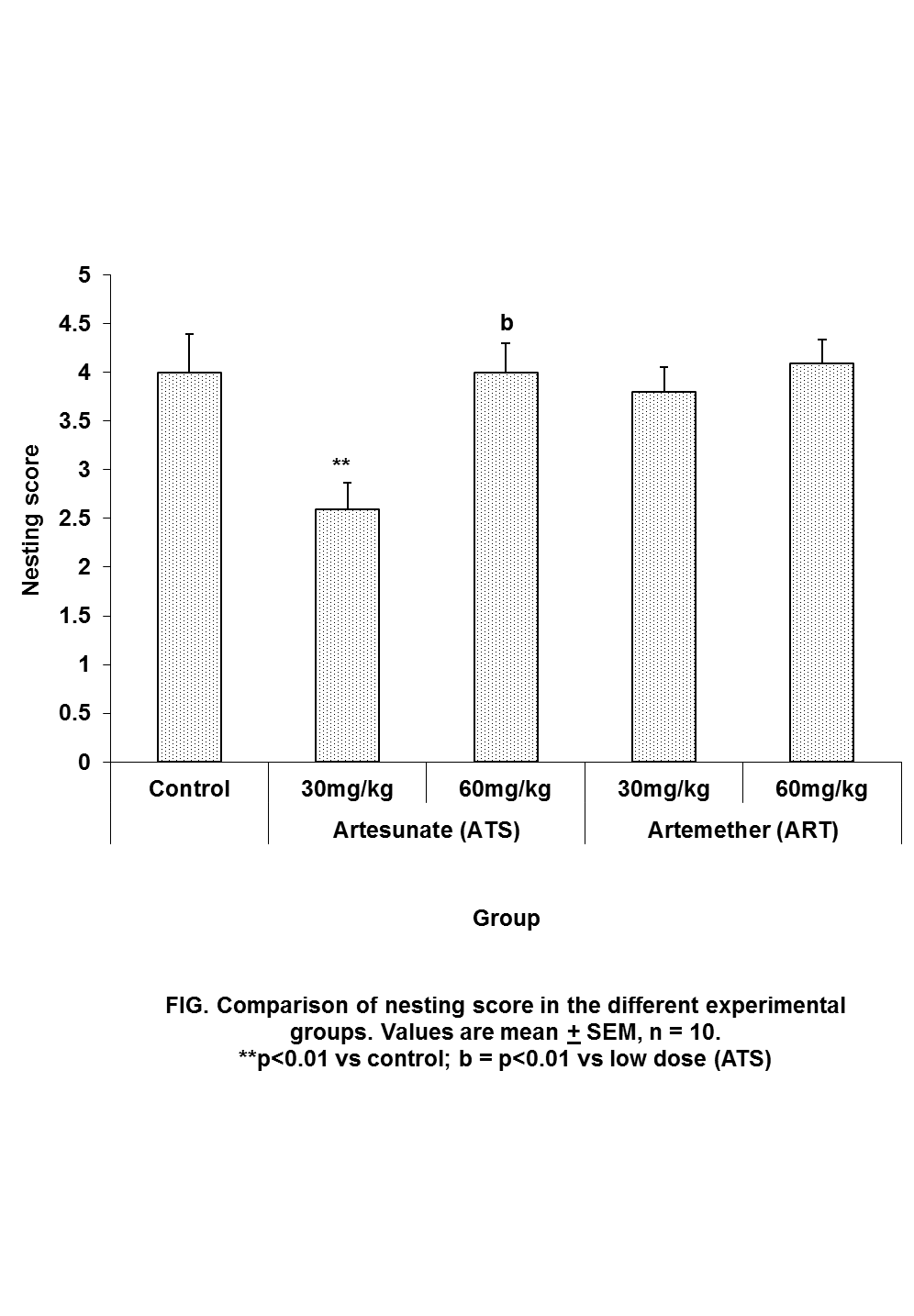 | Figure 1. Comparison of nesting score in mice following oral administration of 30mg/kg and 60mg/kg of Artesunate (ATS) and Artemether (ART) |
 | Figure 2. Comparison of the latency of tail flick in mice following oral administration of 30mg/kg and 60mg/kg of Artesunate (ATS) and Artemether (ART) |
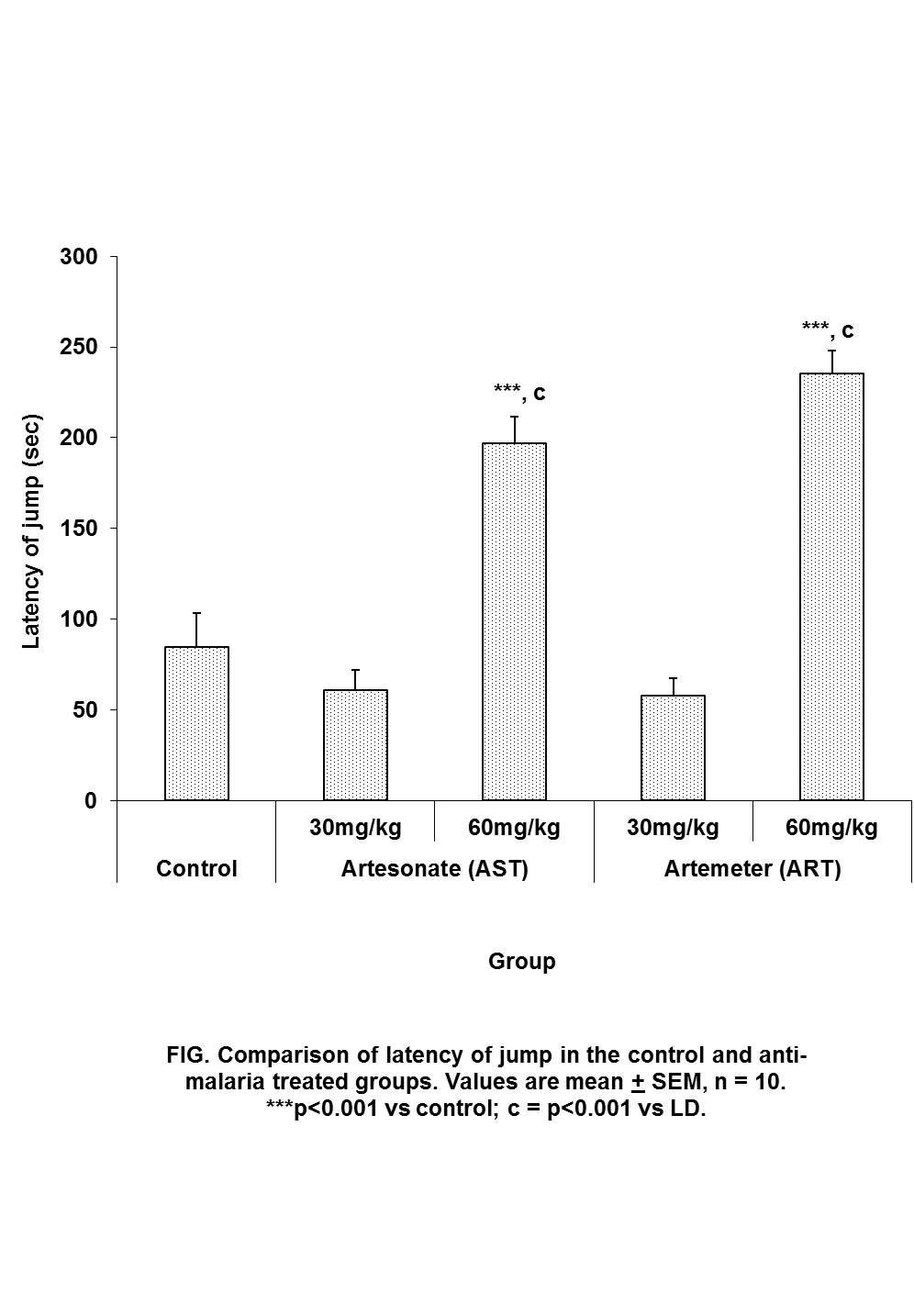 | Figure 3. Comparison of the latency of jump in hot plate test in mice following oral administration of 30mg/kg and 60mg/kg of Artesunate (ATS) and Artemether (ART) |
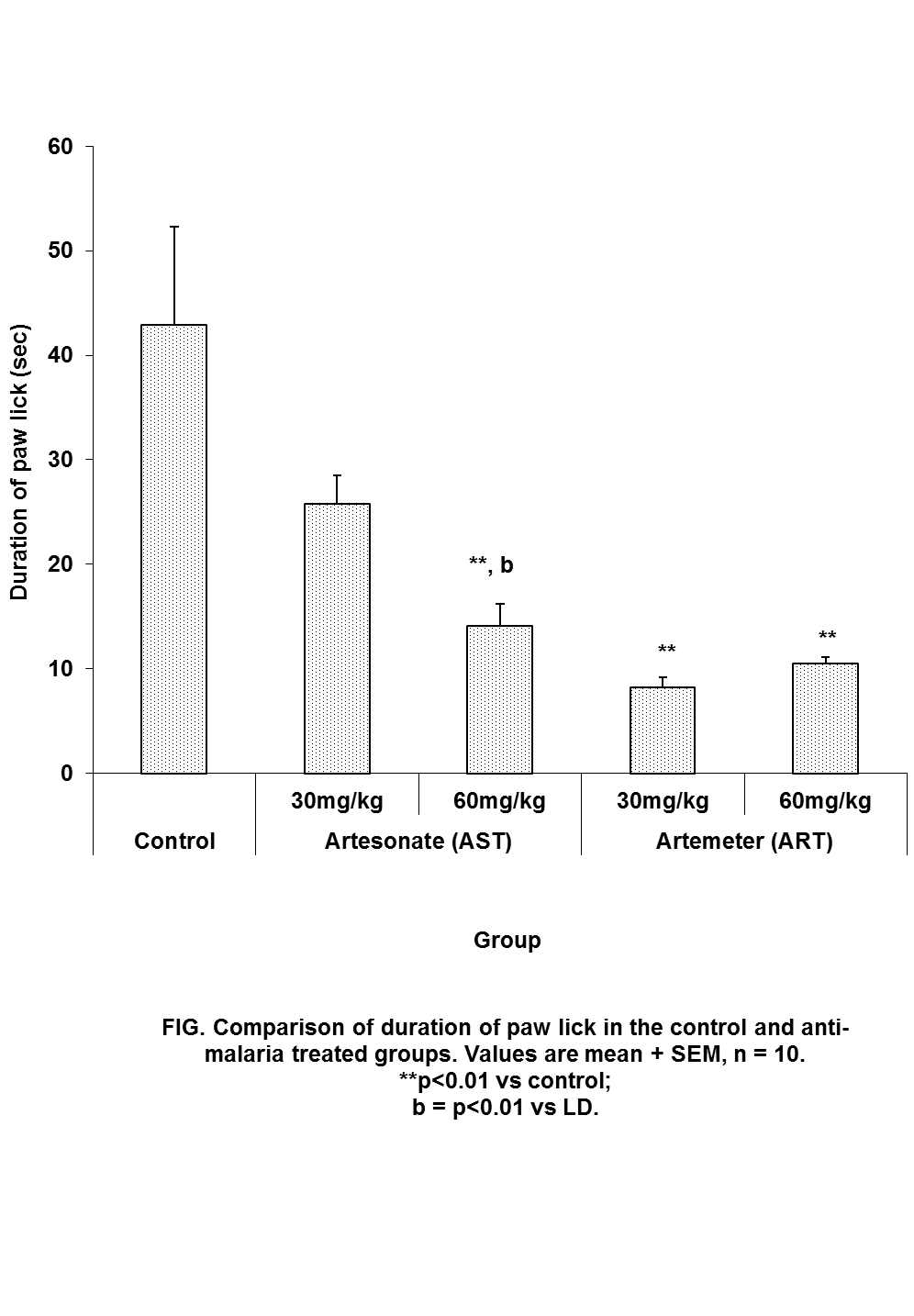 | Figure 4. Comparison of the duration of paw lick in hot plate test in mice following oral administration of 30mg/kg and 60mg/kg of Artesunate (ATS) and Artemether (ART) |
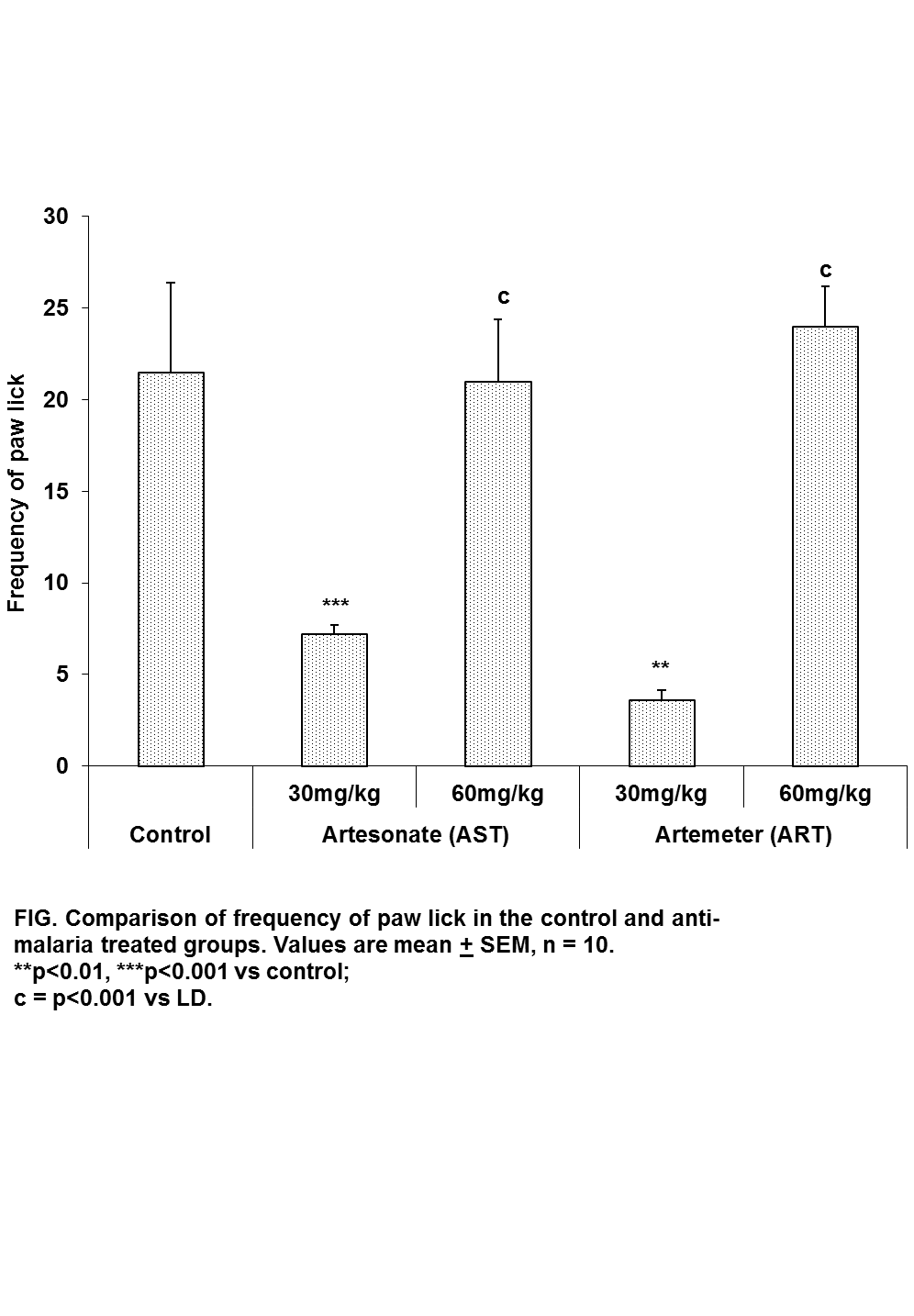 | Figure 5. Comparison of the frequency of paw lick in hot plate test in mice following oral administration of 30mg/kg and 60mg/kg of Artesunate (ATS) and Artemether (ART) |
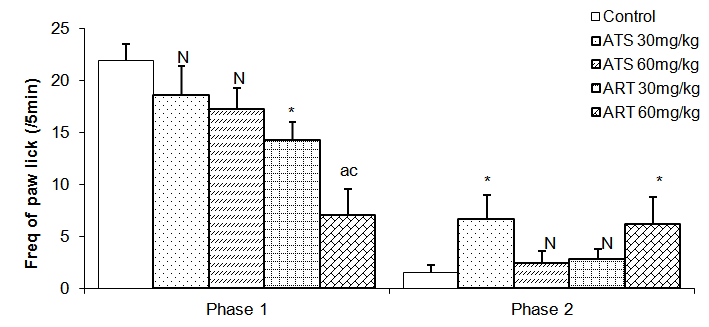 | Figure 6. Comparison of the frequency of paw lick in phase 1 and 2 formalin test in mice following oral administration of 30mg/kg and 60mg/kg of Artesunate (ATS) and Artemether (ART) |
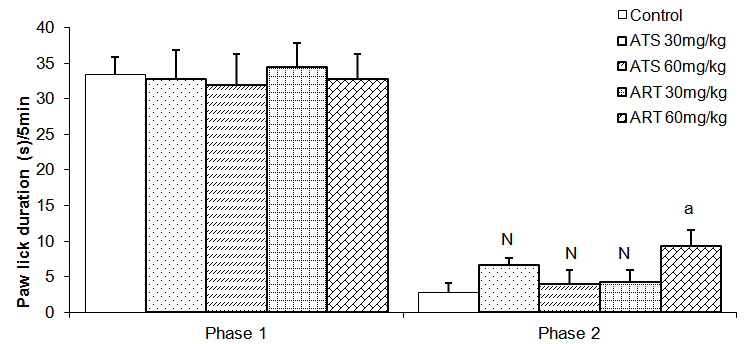 | Figure 7. Comparison of the frequency of paw lick in phase 1 and 2 formalin test in mice following oral administration of 30mg/kg and 60mg/kg of Artesunate (ATS) and Artemether (ART) |
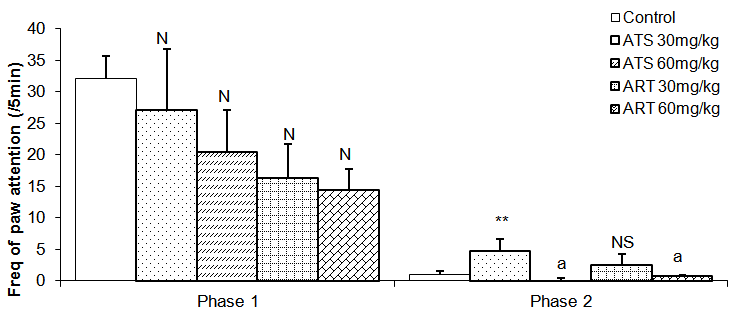 | Figure 8. Comparison of the frequency of paw attention in phase 1 and 2 formalin test in mice following oral administration of 30mg/kg and 60mg/kg of Artesunate (ATS) and Artemether (ART) |
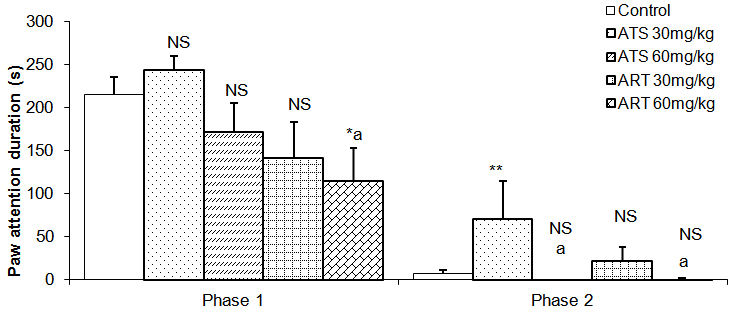 | Figure 9. Comparison of the paw attention duration in phase 1 and 2 formalin test in mice following oral administration of 30mg/kg and 60mg/kg of Artesunate (ATS) and Artemether (ART) |
4. Discussion
4.1. Social Behavior
- Social behavior was assessed in this study by nesting score. Nesting score was significantly lower than control only in artesunate (30mg/kg). This appearslike ‘artifactual’ finding since mice in the higher group of Artesunate were not affected. Social behaviour is controlled by the higher centres especially the medial temporal cortex, midline and medial subcortical cortical structures including amydala and hippocampus. In particular, hippocampal lesioned mice were shown to exhibit very poor nesting score (Deacon, 2012).The reason why social behaviour was not affected in this study could be due to the fact that neurons in the higher centre are not as susceptible to artemisinin neurotoxicity as the brainstem as reported by an earlier study (Shumucket al., 2002). This finding is further supported by an earlier study ours where there was no impairment in the Morris water maze experiment following prolonged administration of high doses of oral artemether (Davies et al., 2012)
4.2. Comparative Effects of Artesunate and Artemether on Pain Perception
- The animal models of physiological pain include tail-flick and hot plate tests and formalin induced neurogenic response (Ito et al., 2001). The tail flick response is thought to be a spinally mediated reflex in that at least, it persist after section or cold block of upper parts of the spinal cord (Irwin et al., 1951; Bonnycastle et al., 1953; Sinsclair et al., 1988) and the hot plate paw- licking are supraspinally organized behaviour, whereas the neurogenic response of formalin induced behaviour reflects activation of C-fibers prior to any afferent nociceptors (Ito et al., 2001)All three models were employed in this study. There are two variant of the tail flick test. One consists of applying radiant heat to a small surface of the tail. The other involves immersing the tail in water at a predetermined temperature. The second variant was used in this study. The latency of tail-flick was defined as the time it takes for the animals to flick its tail following immersion in hot water. Longer tail flick latencies would indicate a higher pain threshold and therefore decreased pain or analgesic effect. Shorter tail flick latencies conversely indicate lower pain threshold and thus increased pain perception or hyperalgesic effect. Similarly, longer latencies of hind-paw licking indicate analgesic effect. Subcutaneous formalin injection into the distal surface of the rat or mouse hind paw elicits two distinctively and quantifiable nociceptive behaviour; namely,flinching/shaking and biting/licking of the injected paw (Dubuisson and Dennis, 1977; Tyolsen et al., 1992). This formalin induced nociceptive behaviour shows an early and late phase. The early phase which starts immediately following injection of formalin only last for 5 minutes and is probably due to direct chemical stimulation of nociceptors (acute pain). The second phase, which last 20 to 40 minutes starts approximately 15 to 20 minutes following formalin injection and experimental data suggest that peripheral inflammatory processes are involved (Haley et al., 1989). This second phase of pain denotes chronic pain (Osim, 2003). The formalin test differs from most other nociceptive tests, such as the hot plate, tail flick and tail pinch tests, in that it enables evaluation of analgesic activity towards moderate, continuous pain generated by injured tissues. As a result, it has been suggested that this test provides a more valid model for clinical pain compared to the threshold or reflex tests such as the hot plate and tail flick tests (Dubuisson and Dennis, 1977; Abbott et al., 1981).Normally, the fine afferent C- and A- delta fibres are activated by brief high intensity stimuli which induce little or no tissue damage. However, during inflammation induced by a mild tissue damage or infection, the afferent fibres can be activated by lower intensity stimuli and the pain produced differs in quality and may be more persistent. C fibres mediate this persistent pain often referred to as slow, chronic or second phase pain whereas A-delta fibres mediates fast pain or acute pain. The formalin test involves an early phase which is caused by the ability of formalin to induce acute pain and a late phase based on its ability to induce chemical responses in tissues resulting in inflammatory responses and slow pain. This late phase involves a phase of inflammation in which a variety of chemical mediators for example, bradykinin, prostaglandin, serotonin and histamine alter the functions of peripheral afferent fibres. Hence phase 1 is pain produced by direct nerve stimulation and phase 2 is an inflammation induced pain.Formalin test: phase 1Reduction inpaw lick frequency in groups treated with 30mg/kg and 60mg/kg of artemether indicates that this drug possesses analgesic activity. This point is also buttressed by the fact that mice treatedwith 60mg/kg of artemether had reduced paw attention duration in addition to reduced paw lick frequency mentioned above. Furthermore, this analgesic activity appears to be more pronounced at 60mg/kg than 30mg/kg of artemether.Formalin test: phase 2Contrary to the effects seen in phase 1, artemether at 60mg/kg appears toenhance pain in phase 2 as shown by increase in frequency and duration of paw lick. Similarly, animals treated with 30mg/kg of artesunate also demonstrated this pain enhancement in phase 2 by increased paw lick frequency, paw attention frequency and paw attention duration.These effects were however, not seen in mice treated with 60mg/kg of artesunate.Hot plate testReduced duration of paw licks in the 60mg/kg artesunate, 30mg/kg artemether and 60mg/kg groups indicate anti-nociceptive activity of these drugs. Increased latency of jump seenin Artesunate (60mg/kg) and Artemether (60mg / kg) groups tends to suggest increasing analgesic activity with increasing dosage especially with artemether. It is unclear why frequency of paw licks was seen in the groups treated with 30mg/kg of either drug not in those treated with 60mg/kg.Tail flick testTail flick latency was significantly increased in mice treated with 60mg/kg of Artemether. This further indicates that Artemether analgesic effect tend to be more pronounced with increased dosage.As earlier stated, formalin phase 1, hotplate test and tail flick test all measure acute pain while formalin phase 2 measures chronic pain. Artemether cause suppression of parameters that indicate acute pain. This effect was also seen in Artesunate at 60mg/kg but was not as pronounced as that of Artemether. On the other hand, Artemether caused enhancement in the parameters that indicate slow pain. Artesunate caused a similar effectat 30mg/kg but not at 60mg/kg of Artesunate. This finding is difficult to interpret. However, it is possible that small doses of Artesunate may cause chronic pain enhancement while larger doses may cause suppression of acute pain. The mechanism by which these drugs suppressed acute but enhanced chronic pain is not clear. At present, this is the only study assessing the effect of artemisinin on nociception. It is hoped that more studies will be done in the future in this regard. In conclusion, oral artemetherand oralartesunate (to a lesser extent) at the dose of 60mg/kg caused suppression of acute pain. However, both drugs appeared to enhance slow pain. Though the findings of this study may not be directly extrapolated to human but it is important to bear this in mind especially with the possibility that these drugs may someday be used as anti-cancer drugs. Both drugs did not have effect on social behaviour.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML