-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Neuroscience
p-ISSN: 2326-1226 e-ISSN: 2326-1234
2013; 2(2): 19-23
doi:10.5923/j.neuroscience.20130202.01
Left Prefrontal Cortex Contributes to Motor Imagery: A Pilot Study
Ina M. Tarkka1, Dobrivoje S. Stokic2
1Department of Health Sciences, University of Jyväskylä, Jyväskylä, Finland
2Center for Neuroscience and Neurological Recovery, Methodist Rehabilitation Center, Jackson, MS, USA
Correspondence to: Ina M. Tarkka, Department of Health Sciences, University of Jyväskylä, Jyväskylä, Finland.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Transcranial magnetic stimulation (TMS) over the motor cortex during motor imagery results in increased amplitudes of motor evoked potentials (MEPs) in muscles specific to the imagined movement. Functional MRI studies demonstrate that motor imagery involves a widespread neural network including prefrontal and parietal areas. The purpose of this pilot TMS study was to explore whether the left prefrontal cortex (PFC) is an active part of the motor imagery network. MEPs were recorded in 5 healthy subjects in tibialis anterior (TA) muscles during the imagined right ankle dorsiflexion while the neural processes in the left PFC were disturbed by a single TMS pulse 300ms prior to the leg area stimulation with TMS. Motor imagery alone significantly increased MEP amplitudes in the target TA muscle compared to rest(440% on average, p=0.004). The left PFC stimulation prior to test stimulus significantly reduced the facilitation of MEPs during motor imagery (326%, p=0.031) butleft PFC stimulation had no effect when delivered during rest (103%). This suggests a functional link between the left PFC and sensorimotor cortex actively involved in motor imagery. Appropriately timed TMS over the left PCF most likely disturbed the kinesthetic working memory required for maintaining motor imagery.
Keywords: Prefrontal Cortex, Motor Cortex, Transcranial Magnetic Stimulation, Kinesthetic Working Memory, Motion Imagery, Ankle Dorsiflexion
Cite this paper: Ina M. Tarkka, Dobrivoje S. Stokic, Left Prefrontal Cortex Contributes to Motor Imagery: A Pilot Study, Research in Neuroscience , Vol. 2 No. 2, 2013, pp. 19-23. doi: 10.5923/j.neuroscience.20130202.01.
Article Outline
1. Introduction
- Imagining motor actions, i.e. performing covert movements, is known to modulate corticospinal excitability [3, 4, 12]. Various neuroimaging studies utilizing PET or functional MRI support the idea that mental simulation of movements involves congruent brain areas that are active during action execution and during observation of actions performed by others[1, 2, 11, 16]. The motor evoked potentials (MEPs) evoked by a single pulse transcranial magnetic stimulation (TMS) are higher in amplitude in the target muscle when subjects imagine muscle contractions compared to rest and this effect is specific to the muscles involved in the imagined movement[3, 4, 12]. Moreover, there is reportedly a positive correlation between the MEP amplitude increase and the individual imagery ability[10, 17]. Besides a transient enhancement in the excitability of the involved corticospinal motor pathway, motor imagery also causes a reduction in the intracortical inhibition within the primary motor cortex (M1) [14]. These excitability changes have not been ascribed to theglobal arousal in the brain and spinal cord but instead to the subliminal and focused changes in very specific areas involved in cognitive processes, such as short-term maintenance of kinesthetic information during motor imagery. This kinesthetic working memoryinvolves a widespread neural network and activates also the ventrodorsal stream of the left hemisphere as shown e.g. by Fiehler et al. 2008 [5]. The network includes the dorsolateral prefrontal cortex (PFC) and it may play a role in maintaining motor imagery as the representations of a given motor act are internally rehearsed during imagery tasks.Considerable evidence indicates that the PFC-mediated cognitive control of behavior covers a wide range of specific cortical operations[6, 8, 9]. In the present study, we explore short-term kinesthetic memory function in the PFC. Memory functions are represented by distributed, interactive, and overlapping networks of neurons in the association cortex. According to Fuster[7], the frontal association cortex contains executive cognits, including also parasensory and premotor cortex cognits representing simple percepts or motor acts. In addition, posterior and frontal networks are associated by long reciprocal cortico-cortical connections forming a basis for the network[7]. The dorsolateral PFC is presumably a part of the explicit working memory network involved in establishing a novel association between visual cues and motor commands. Trainedvisuomotor skills appear to increase specifically the left hemisphere activity[8] and left M1dominance for motor imagery was also suggested in healthy subjects[15]. This shift to the left hemisphere has been observed regardless of the side of the practiced motor activity suggesting the left hemispheric dominance in storage of visuomotor skills[8].The PFC regions that connectto the primary somatosensory cortex (S1) were recently used as the targets for TMS in a working memory study[13]. The results showed that the prefrontal topdown suppression via the middle frontal gyrus-S1 link plays a role in the maintenance oftactile working memory. Although it is apparent that aroused attention and kinesthetic working memory contribute to motor imagery, it is unclear if the left PFC has any role in imagery. To explore this, we utilized in this pilot study the TMS for selective and transient functional silencing of the left dorsolateral PFC during motor imagery and assessed its effect on the responses to M1 stimulation. Based on the timing presented by Savolainen et al.[13], we used a 300ms delay between the left PFC and M1 stimulation. Our hypothesis was that the involved kinesthetic working memory processes maintaining imagery within the left PFC will be disturbed by the TMS and will therefore result in lesser facilitation of M1 during motor imagery.
2. Participants and Methods
- The study participants were five healthy adults who reported no neurological symptoms (4 males, 1 female, mean age 56±6 years). They signed informed consentand the study was approved by the local institutional review board for human research. Subjects were seated comfortably in a custom-made chair, kept their eyes open, and were asked to imagine smooth ankle dorsiflexion (DF) motion during the presentation of a visual cue (2 s on, 2 s off) displayed on the monitor about 1 m in front of them.They were asked to use kinesthetic rather than visual imagery. A thorough practice session was performed before the experiment with the verbal guidance of the researcher.Bipolar surface EMG was recorded with 10mm diameter electrodes placed bilaterally over TA muscles (impedance below 5 kΩ) using a commercial EMG equipment.The TMS paradigm was set up utilizing two different coils and two stimulators (Magstim 200™). First, the 90mm round coil was placed over the optimal scalp location for evoking right TA responses (Cz-TMS, approx. 1 cm in front of Cz along the midline). The stimulus intensity was set at 120% of the individual resting motor threshold (85%±10% of the maximum stimulator output). The second coil (figure-8 D70 mm) was placed over F3 location of the International 10-20 System (7 cm anterior and 3 cm lateral from Cz) to optimally stimulate the left dorsolateral PFC (F3-TMS). The F3-TMS stimulus intensity was set to 10% less than the Cz-TMSaimed at activating the right TA muscle (mean 75%±10% of the maximum stimulator output) and delivered 300msearlier. Once the individual stimulation locations and intensities were selected, they were kept unchanged throughout each individual’s study with custom-made 3D coil holders. Data collection protocol consisted of 1) Cz-TMS during relaxation (baseline, CTRL_1), 2) Cz-TMS during imagined DF (IMG) to assess the degree of facilitation in M1, 3) combined F3-TMS and Cz-TMS 300ms apart during imagined DF (PFC_IMG) to assess the contribution of the left PFC to motor imagery (study objective), and 4) combined F3-TMS and Cz-TMS 300ms apart during relaxation to assess the influence of left PFC on M1 in the absence of motor imagery (CTRL_2). A series of additional control experiments was carried out in one subject (combined F3-TMS and Cz-TMS 0ms apart during imagined DF to assess the timing of interference effect, and combined F3-TMS and Cz-TMS 300ms apart during 2 s relaxation portion of the imagined DF task to assess the influence of the left PFC on M1 when the working memoryis temporarily disengaged during the imagery task). The TMS was manually delivered about every 10 s corresponding to the middle of the visual cue with 8-12 trials recorded for each condition. Individual MEPsfor each condition were averaged and submitted to statistical analysis. The changes in MEPs in different conditions were expressed as a percent change relative to the respective control (%). Data were normally distributed and no MEP outliers were removed. Paired t-test was used for determining significant differences between different conditions (p<0.05), Cohen’s effect sizes were calculated, and no corrections for multiple comparisons were made due to the preliminary nature of the investigation.
3. Results
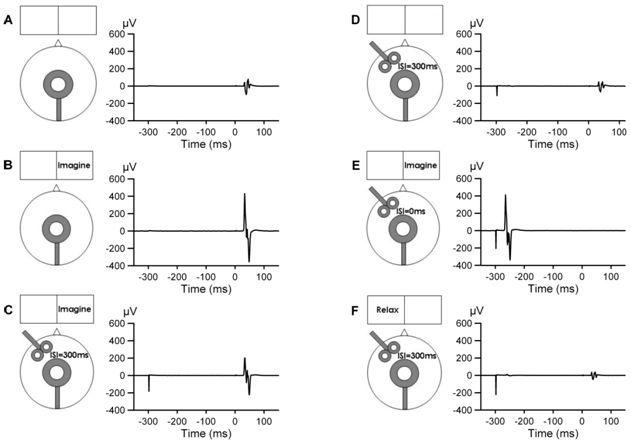
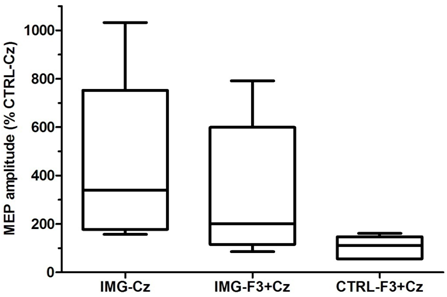
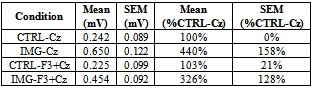
- The MEP amplitudes in the right TA muscle significantly increased to an average of 440% (p=0.004) during imagined DF movements compared to relaxation (Table 1, IMG-Cz). This remarkable facilitation was significantly diminished to 326% (p=0.031) when the TMS to left PFC was delivered 300ms prior to M1 stimulation (Table 1, IMG-F3+Cz).When analyzing the effect of imagery to rest, Cohen’s d was 1.74 indicating about 80% probability of increased amplitude whereas during PFC stimulation while performing imagery the effect size was less, 1.01.Therefore our hypothesis was confirmed, i.e., the left PFC stimulation decreased the facilitation of M1 during motor imagery. The effect sizes of these amplitude changes differ by 0.7, which could be considered at least moderate (effect size 0.8 is often considered large). In the control experiment, the PFC stimulation during relaxation left MEPs unchanged (103%), clearly indicating that there is no effect of the left hemisphere PFC on M1 in the absence of motor imagery. An example of the averaged individual waveforms from the main experiment is shown in Fig. 1A-C. The additional control experiments performed in the same subject illustrate no effect on MEPs when the combined left PFC and Czstimulations were delivered during relaxation (D), delivered simultaneously during motor imagery (E), or delivered 300ms apart during 2s relaxation portion of the imagined task (F). The box and whiskers plot (Fig. 2) illustrates the group data for the three main experimental conditions.
4. Discussion
- Motor imagery is thought to be governed by top-down cortical control mechanisms. Here we applied TMS at two distinct locations to explore the functional connectivity between the left dorsolateral PFC and M1 during motor imagery. By delivering TMS over the left PFC 300ms prior to M1 stimulation, we were able to disturb the motor imagery process, as indicated by decreased corticospinal excitability. The obtained change in MEP amplitudes due to PFC stimulation during imagery was at least moderate, close to large, in terms of effect size. This stimulation set-up was chosen based on the finding that a specific site in the middle frontal gyrus within Broadmann areas 9 and 46 has an anatomic link to primary sensory area[13]. It was previously proposed that, via this link, the middle frontal gyrus contributes to top-down control of tactile working memory. Maintenance of kinesthetic working memory is necessary for motor imageryandit presumably involves a diverse neural network with all parts of it not well known[5, 9, 16]. Kinesthetic encoding of hand movements was shown to activate sensory and premotor areas as well as anterior middle frontal gyrus and PFC[5]. We postulate that TMS over the left dorsolateral PFC disturbed the kinesthetic working memory required for maintaining the requested motor imagery, which led to decreased facilitation in M1. Although only 300ms delay between the PFC and M1 was explored, we anticipate that shorter or longer delays mayalso reveal contribution of the left PFC to motor imagery. However, the precise timing of shorter delays may be difficult to ascertain due to variability in subject’s performance of motor imagery. It is clear that our small sample serves only as pilot experiment and the complex control what PFC attributes to M1 during motion imageryneeds to be more carefully studied. Yet the absence of interference effect upon simultaneous delivery of TMS to the left PFC and M1 during motor imagery re-affirms our main findings. That our findings are specific to motor imagery is suggested by the absence of interference effect in additional control experiments done at the same delay in relaxation, either entirely before engaging in motor imagery (Fig.1 D) or in between blocks of motor imagery (Fig.1 F).
5. Conclusions
- Our results suggest that there is a clear a functional link between the left dorsolateral PFC and sensorimotor cortex in healthy subjects which contributes to motor imagery. This justifies pursuing further studies with more precise neuronavigationin order to precisely map the involved PFC areas and their extent. Also further studies should explore whether this link is dysfunctional in pathological conditions (e.g., stroke) and which type of lesions limit the use of imagery for restoration of motor functions.
ACKNOWLEDGEMENTS
- The authors thank the subjects of the study. This work was supported in part by the University of Jyväskylä International Mobility Grant to Dr. I. M. Tarkka and by the Wilson Research Foundation, Jackson, MS
References
| [1] | Beisteiner, R., Hollinger, P., Lindinger, G., Lang, W., Berthoz, A. Mental representations of movements. brain potentials associated with imagination of hand movements. Electroencephalogr Clin Neurophysiol. 1995, 96:183-93. |
| [2] | Decety, J., Perani, D., Jeannerod, M., Bettinardi, V., Tadary, B., Woods, R., Mazziotta, J. C., Fazio, F. Mapping motor representations with positron emission tomography. Nature. 1994, 371:600-2. |
| [3] | Fadiga, L., Buccino, G., Craighero, L., Fogassi, L., Gallese, V., Pavesi, G. Corticospinal excitability is specifically modulated by motor imagery: A magnetic stimulation study. Neuropsychologia. 1999, 37:147-58. |
| [4] | Fadiga, L., Craighero, L. Electrophysiology of action representation. J Clin Neurophysiol. 2004, 21:157-69. |
| [5] | Fiehler, K., Burke, M., Engel, A., Bien, S., Rosler, F. Kinesthetic working memory and action control within the dorsal stream. Cereb Cortex. 2008, 18:243-53. |
| [6] | Fuster, J. M. The prefrontal cortex--an update: Time is of the essence. Neuron. 2001, 30:319-33. |
| [7] | Fuster, J. M. Cortex and memory: Emergence of a new paradigm. J Cogn Neurosci. 2009, 21:2047-72. |
| [8] | Halsband, U., Lange, R. K. Motor learning in man: A review of functional and clinical studies. J Physiol Paris. 2006, 99:414-24. |
| [9] | Ikkai, A., Curtis, C. E. Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia. 2011, 49:1428-34. |
| [10] | Lebon, F., Byblow, W. D., Collet, C., Guillot, A., Stinear, C. M. The modulation of motor cortex excitability during motor imagery depends on imagery quality. Eur J Neurosci. 2012, 35:323-31. |
| [11] | Macuga, K. L., Frey, S. H. Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. Neuroimage. 2012, 59:2798-807. |
| [12] | Rossini, P. M., Rossi, S., Pasqualetti, P., Tecchio, F. Corticospinal excitability modulation to hand muscles during movement imagery. Cereb Cortex. 1999, 9:161-7. |
| [13] | Savolainen, P., Carlson, S., Boldt, R., Neuvonen, T., Hannula, H., Hiltunen, J., Salonen, O., Ma, Y. Y., Pertovaara, A. Facilitation of tactile working memory by top-down suppression from prefrontal to primary somatosensory cortex during sensory interference. Behav Brain Res. 2011, 219:387-90. |
| [14] | Stinear, C. M., Byblow, W. D. Modulation of corticospinal excitability and intracortical inhibition during motor imagery is task-dependent. Exp Brain Res. 2004, 157:351-8. |
| [15] | Stinear, C. M., Fleming, M. K., Byblow, W. D. Lateralization of unimanual and bimanual motor imagery. Brain Res. 2006, 1095:139-47. |
| [16] | Vry, M. S., Saur, D., Rijntjes, M., Umarova, R., Kellmeyer, P., Schnell, S., Glauche, V., Hamzei, F., Weiller, C. Ventral and dorsal fiber systems for imagined and executed movement. Exp Brain Res. 2012, 219:203-16. |
| [17] | Williams, J., Pearce, A. J., Loporto, M., Morris, T., Holmes, P. S. The relationship between corticospinal excitability during motor imagery and motor imagery ability. Behav Brain Res. 2012, 226:369-75. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML