-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Marine Science
p-ISSN: 2163-2421 e-ISSN: 2163-243X
2021; 9(1): 1-10
doi:10.5923/j.ms.20210901.01
Received: Mar. 31, 2021; Accepted: Apr. 23, 2021; Published: May 15, 2021

Symbiodinium Distribution Patterns in Millepores in the Caribbean: South Water Cay, Belize and San Salvador, The Bahamas
Mara Waechter Schwiesow1, Allison Moreno Samayoa1, 2, Jocelyn Torres1, Amanda Leimbach1, Glorisette Santiago-Rivera1, Craig S. Tepper1
1Department of Biology, Cornell College, Mt Vernon, IA, USA
2Cancer Biology, University of Arizona, Tucson, AZ, USA
Correspondence to: Craig S. Tepper, Department of Biology, Cornell College, Mt Vernon, IA, USA.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Symbiosis with Symbiodinium (Dinoflagellata, Chromalveolata) plays a major role in the survival of numerous coral species. Different Symbiodinium clades provide their hosts with different physiological advantages. However, much of the research concerning coral-Symbiodinium associations has focused on scleractinian corals, while reef-building Millepores (fire coral) have mostly been ignored. We examined the Millepore-Symbiodinium relationship at two thermally different sites in the Caribbean: San Salvador, The Bahamas and South Water Cay, Belize. Our results indicate that there is a difference in symbiont dominance between the two sites. Millepores residing in the cooler sea surface temperatures (SST) of The Bahamas are Symbiodinium clade B dominant (100%) and are not showing signs of bleaching. However, Belize Millepores experiencing warmer SST are mostly Symbiodinium clade A dominant (72%) and are not showing signs of bleaching. Some clade B dominant Belize Millepores (28%) are showing signs of bleaching. These findings are corroborated by a reversal in symbiont dominance of Belize Millepores residing at deeper depths; most are Symbiodinium clade B dominant (80%) and none of these colonies showed signs of bleaching. Our results appear to correlate with the temperature difference between the two sites, and suggest Belize Millepores may be experiencing thermal stress events more frequently. The relationship between specific Symbiodinium clades and their coral hosts may provide the coral with a mechanism to cope with increased thermal stress due to global warming.
Keywords: Symbiodinium, Millepore, Clade dominance
Cite this paper: Mara Waechter Schwiesow, Allison Moreno Samayoa, Jocelyn Torres, Amanda Leimbach, Glorisette Santiago-Rivera, Craig S. Tepper, Symbiodinium Distribution Patterns in Millepores in the Caribbean: South Water Cay, Belize and San Salvador, The Bahamas, Marine Science, Vol. 9 No. 1, 2021, pp. 1-10. doi: 10.5923/j.ms.20210901.01.
Article Outline
1. Introduction
- Coral reefs host more than 25% of all known marine species [1], but recent estimates have projected a 70-90% decline in coral reefs by 2050 due to climate change [2]. The loss of coral cover not only decreases biodiversity, but also has negative impacts on local and global economies. Reefs generate revenue by supporting coastal development through the fishing and tourism industries, which in turn creates jobs. Coral reefs have an estimated net worldwide economic value of over $30 billion USD per year [1]. Small tropical nations especially rely on the tourism trade. In Bermuda, for example, 12% of the yearly gross domestic product (GDP) depends on local reefs [3]. In Belize, coral reefs contribute up to 15% of the GDP [4]. Coral reefs also comprise a major food source for over 500 million people around the world [1]. Therefore, gaining a better understanding of the impact of climate change induced stressors on coral reefs is of the utmost importance.Global warming has been linked to coral bleaching, and is causing devastating effects on coral reefs and the biodiversity they support [5]. Coral bleaching usually occurs when sea surface temperatures (SST) reach 30°C or above for 3 to 4 weeks, which in most cases only requires a 1 to 2°C water temperature increase above ambient temperatures [6]. Since the 1980s, there have been three pan-tropical bleaching events (1998, 2010, and 2015-2016) along with numerous regional mass bleaching events, resulting in coral death and decreased coral cover [7]. Hughes et al. [7] noted that with each successive bleaching event, fewer reefs escaped unscathed. During the 1998 bleaching event, 45% of the surveyed reefs survived with no bleaching, but during the 2016 event, only 9% of the surveyed reefs escaped with no bleaching [7]. Bleaching is caused by the loss of symbiotic algae (Symbiodinium) that reside in the corals’ tissue [8]. Symbiodinium can provide up to 95% of the corals’ nutritional needs, so losing symbionts during bleaching weakens the corals and makes them more susceptible to disease and future climatic events [9].Symbiodinium, commonly known as zooxanthellae, are a group of photosynthetic dinoflagellates that form symbiotic relationships predominantly with reef-building corals, as well as with a wide range of marine invertebrates including Porifera, other Cnidarians, Platyhelminthes, and Mollusca. The vibrancy of coral colors comes from the photosynthetic pigments of the algae they house [8]. There is a high degree of diversity among Symbiodinium, and they are genetically classified into nine clades, A through I, with numerous subclades [10]. Symbiodinium tend to have a high specificity for their coral hosts, and hosts exhibit a genetic preference for certain clades of Symbiodinium. For example, Scleractinia (stony) corals form symbiotic interactions with Symbiodinium clades A, B, C, D, and F, while Hydrozoan (fire) corals predominantly associate with Symbiodinium clades A, B, and C [10]. Although Symbiodinium-coral interactions are specific, corals that form associations with Symbiodinium can contain either one clade, or one dominant with multiple background clades [11]. Baker and Romanski [12] found that 72% of the coral species they examined had multiple clades and subclades, indicating that Symbiodinium-host associations are flexible.Corals that host Symbiodinium grow on shallow reefs where there is more light, and temperatures are already close to the tipping point for bleaching. Symbiodinium are located within organelles called symbiosomes, and photosynthetic products and other nutrients are passed between the host and the symbiont through the symbiosome membrane [13]. The coral, in return, provide the Symbiodinium with protection and exposure to light [14]. However, numerous environmental factors can stress the relationship resulting in a breakdown of symbiosis, loss of the symbionts, and, ultimately, coral bleaching [11]. The physiological mechanisms that lead to the selective loss of symbionts and bleaching may be due to the symbiont’s release of reactive oxygen species (ROS) during photosynthesis [15].Under non-stressful environmental conditions, ROS produced during photosynthesis are mitigated by antioxidants [16]. However, increased irradiance levels lead to increased ROS which overwhelms the symbionts’ photosystems’ ability to scavenge ROS [17]. Wietheger et al. [15] measured the production of ROS in cultured Symbiodinium clades A1, B2, and F1 under thermal and oxidative stress conditions using fluorescent probes, and observed significant increases in ROS in all clades. Oxidation caused by ROS has been postulated to destroy the symbiosome membrane, damage host cell proteins and membranes, and cause the loss of symbionts, resulting in bleaching [18]. However, the hypothesis that ROS are responsible for bleaching is controversial. Nielsen et al. [19] found it unlikely that oxidative stress to host cells was the cause of coral bleaching in Pocillopora damicornis (cauliflower coral). The stress that results in bleaching may provide the opportunity for coral to adapt to changing environmental conditions by allowing coral to alternate between specific symbionts that confer physiological advantages to their hosts. The Adaptive Bleaching Hypothesis (ABH) proposes that stress events provide an opportunity for the host to be repopulated with different symbionts that are better adapted to help the coral survive environmental change [16]. This exchange occurs in one of two ways: either switching (recruitment of new symbionts from the environment) or shuffling (changes in the dominant symbiont present in a coral colony) [20,21,22]. Switching or shuffling symbiont clades allow corals to “select” the symbiotic relationship best suited to cope with environmental stressors, and thus allow corals to better adapt to their current environment.Since it was first proposed, much evidence has supported the ABH. Baker et al. [23] surveyed reefs in Kenya, Mauritius, the Red Sea, Persian Gulf, and Panama during the pre-stress (1995), stress (1997), and post-stress (2001) periods of the 1997-1998 El Niño event. They found that corals that originally contained C as their dominant clade and shuffled clade D from a background clade to a dominant clade were less affected by the increased temperatures than those coral that remained C dominant. They concluded that clade D is more thermally tolerant and the symbiosis could survive periods of thermal stress [23]. Berkelmans and van Oppen [20] found that Acropora millepora, a common hard coral in the Indo-Pacific, could increase their thermal tolerance by 1-1.5°C by changing the dominant Symbiodinium clade from C to D.There is also evidence that Symbiodinium-host interactions may not be as flexible as once believed, but instead are governed genetically, not environmentally. Poland and Coffroth [24] took the aposymbiotic offspring of the Caribbean octocoral (Briareum asbestinum) from a parental colony that associated with Symbiodinium clade B184 and transplanted the juveniles to a similar habitat where the existing B. asbestinum colonies associated with Symbiodinium clade B178. Transplanted juvenile coral polyps formed symbiotic relationships with many different clades, but after about 4 years, the offspring had associated almost entirely with the dominant clade of the parent colonies (B184), despite the transplant location and the resident B. asbestinum colonies containing a different dominant clade [24]. They concluded that the genetics of both the symbiont and the host coral determine the symbiont diversity, not the environmental conditions. Palumbi et al. [25] showed that transplanted Acropora hyacinthus (bush coral) could adapt to thermal stress with no change to their symbiont population. Therefore, the ABH alone may not explain coral’s adaptive mechanism to cope with thermal stress in all situations.The Caribbean offers an ideal environment to study thermal stress in host-symbiont associations because from 1955 to 2016, the Caribbean has experienced an average temperature increase of 0.24°C per decade [26]. These warming trends are sufficient to push coral outside their range of thermal tolerance. There has been extensive research on Symbiodinium-scleractinian (stony coral) interactions in these conditions, but the ubiquitous reef-building Millepores (fire coral) have largely been ignored [27]. Millepora, a genus of hydrozoan coral, are common encrusting corals found throughout the Caribbean [28]. Traditionally, Caribbean Millepores are classified into two species, Millepora alcicornis and Millepora complanata, based on morphology [28]. M. alcicornis are thinly encrusting sheets and branches found mostly at deeper depths with less wave action and M. complanata are thick rigid blades found mostly at shallower depths [28]. However, the Millepores exhibit phenotypic plasticity resulting in the presence of many intermediate growth forms whose taxonomic status is uncertain [27,28,29,30,31]. We have focused our attention on Symbiodinium-Millepore symbiosis at two study sites where yearly SST is different: San Salvador, The Bahamas (22-28°C) [32] and South Water Cay, Belize (26-31°C) [33]. Since coral bleaching generally occurs slightly above 30°C, the corals at the Belize site may be under greater thermal stress due to heightened irradiance levels, whereas the corals residing in Bahamian reefs may be less likely to cross the bleaching threshold, resulting in a possible difference in symbiont population.In a preliminary study with a small sample size, Samayoa et al. [34] found that clade B might offer Millepores a physiological advantage in the cooler SST of the Bahamas, while clade A might be more beneficial to Millepores in Belize with warmer SST. Here we report that Symbiodinium clade dominance in Millepores differs significantly between cooler SST in reefs around San Salvador, The Bahamas compared to warmer SST in reefs close to South Water Cay, Belize. Belize Millepores residing in shallow patch reefs are mostly clade A dominant. However, colonies collected from deeper reefs in Belize are mostly clade B dominant, indicating a change in dominance with regard to depth.Determining whether certain clades of symbionts offer Millepores a thermal physiological advantage will provide insight into future conservation efforts for corals in the Caribbean and worldwide.
2. Methods and Materials
2.1. Collection and Preparation of Millepore Samples
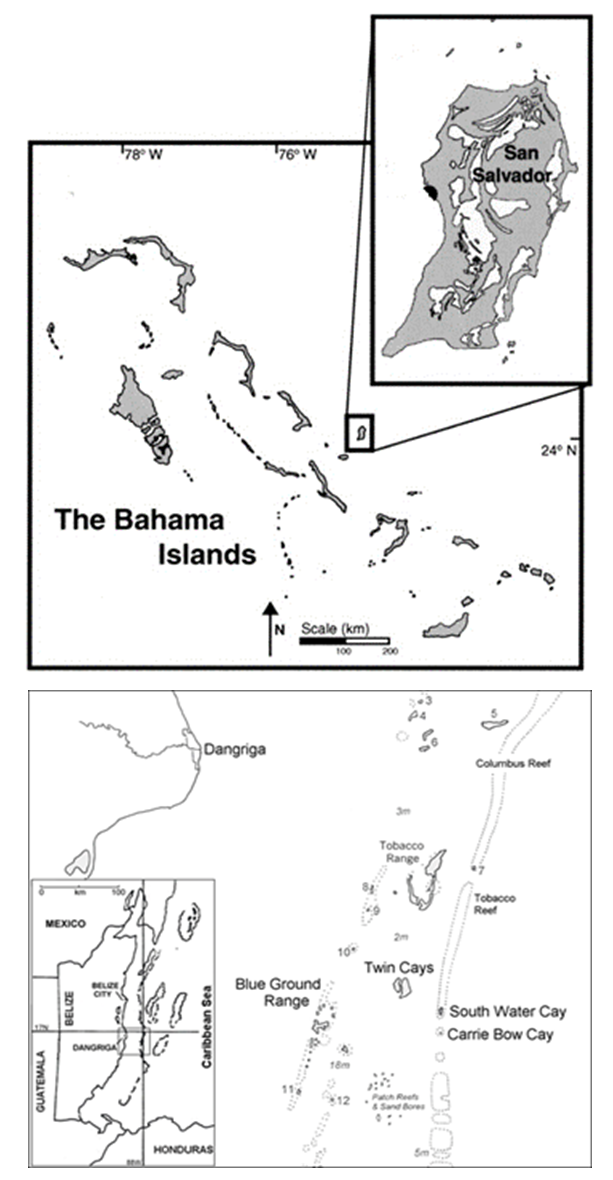
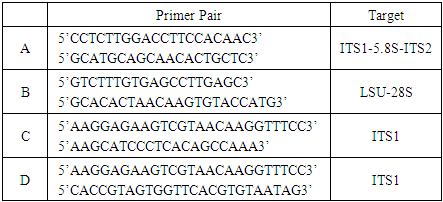
- Millepores used in this study were collected via snorkeling or SCUBA from 2009-2018 on reefs on the west side of San Salvador, The Bahamas (24.1°N, 74.4°E) and from 2013-2019 along the barrier of the South Water Cay Marine Reserve (SWCMR) near South Water Cay, Belize (16.8°N, 88.1°E) (Figure 1). San Salvador is located on the eastern edge of The Bahamas Island Archipelago. Collection sites were shallow (1-5 m) patch reefs located at Lindsay Reef, Rocky Point Reef, Grotto, and French Bay. South Water Cay, which is part of Belize’s largest marine reserve, covers an area of 117,878 acres and is located approximately 15 miles southeast of Dangriga. Millepore samples were collected from shallow (1-5 m) sites around SWCMR located at Whales Shoal, Aquarium, Angel’s Reef, Coral Garden, Tobacco Cay, IZE Reef, and Curlew Reef and deeper (5-24 m) sites at Carrie Bow Fore Reef, Coral Garden, and Aquarium Wall.
2.2. Symbiodinium DNA Isolation
- Symbiodinium DNA was isolated using the procedure of Samayoa et al. (34). Millepore samples were repeatedly blasted with L buffer (100 mM EDTA, 10 mM Tris, pH 7.6) from a 50cc syringe. Removed coral tissue was centrifuged at 3,500 rpm for 10 minutes and the resulting pellet was washed in 10 mL of L buffer and recentrifuged. The tissue pellet was resuspended in 900 μL of L buffer and macerated manually with a tissue homogenizer. The homogenate was centrifuged at 13,000 rpm for 10 minutes and the pellet resuspended in L buffer. Symbiodinium were lysed with SDS (1% w/v) and incubated at 65°C for 60 minutes. Pro K (0.5 mg/mL) was added and the lysate was incubated at 37°C for a minimum of 6 hours. NaCl (0.8 M) and CTAB (1% w/v) were added and the samples incubated at 65°C for 30 minutes. Nucleic acids were ethanol (70%, v/v) precipitated (twice) in sodium acetate (0.3M, pH 5.2) and immediately centrifuged. The pellet was resuspended in dH2O.
2.3. Quantitative PCR
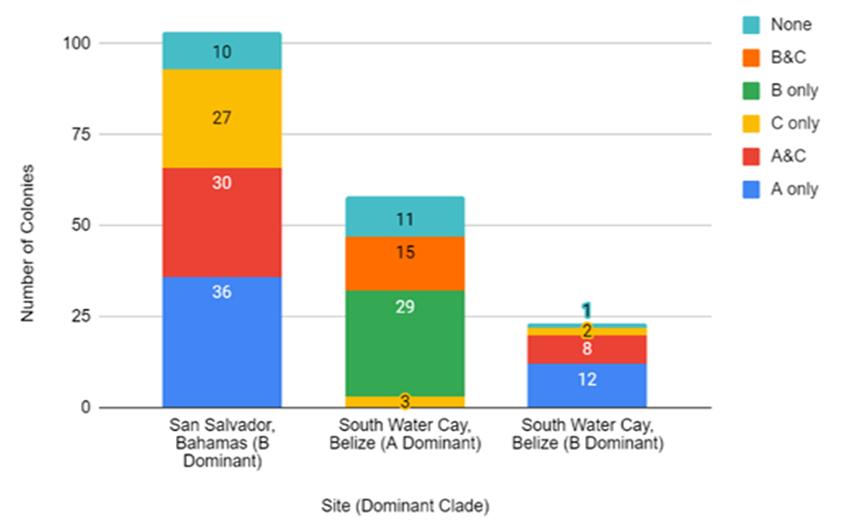
- Four clade-specific Symbiodinium primer pairs [35,36] targeting specific regions of rDNA were used (Table 1). The 20 μL qPCR reaction contained 10 μL of Power SYBR Green Mastermix (Applied Biosystems), 75 nM of clade-specific primer pairs, and 200 ng of Symbiodinium DNA. Amplifications were performed and analyzed on an Applied Biosystems StepOnePlus Real-Time PCR system. The qPCR profile consisted of an initial denaturation of 95°C. for 10 minutes followed by 40 cycles of 95°C for 30 seconds, 58.5°C for 30 seconds and 72°C for 30 seconds. In order to determine if amplification signals represented the amplicon or primer dimers, at the end of each run a melt curve was generated starting at 55°C and increasing the temperature by 0.5°C every 5 seconds until a temperature of 95°C was reached.
|
3. Results
- The same sample set from numerous shallow (1-5 meters) reefs was used to examine the effects of geographic location, morphology, year and temperature, and individual reef location. The individual reefs were in close proximity to each other, and no significant trends were observed between reefs (data not shown).
3.1. Geographic Location
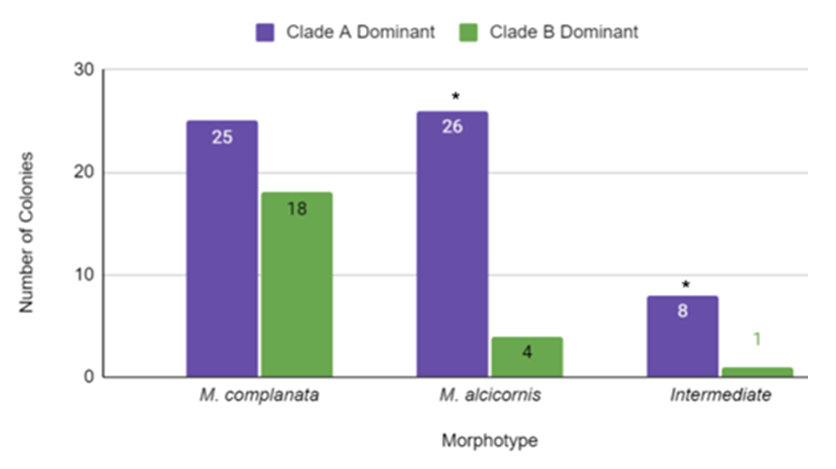
- A total of 184 Millepore colonies were examined for Symbiodinium clades A, B, C, and D from numerous reefs surrounding two study sites, South Water Cay, Belize (N=81) (2013-2017) and San Salvador, The Bahamas (N=103) (2009-2018) (Figure 2). In the Bahamas, Symbiodinium clade B was the dominant clade in all Millepores examined (100%). In Belize, 72% (N=58) of the Millepores were clade A dominant and 28% (N=23) were clade B dominant. Belize Symbiodinium B dominant Millepores seemed to have a greater tendency to bleach (N=5) than Symbiodinium A dominant Millepores in the same areas (Figure 3). No Millepore bleaching was observed in The Bahamas.
3.2. Morphology
- The dominant Symbiodinium clade present in Millepore morphotypes in Belize was also examined (Figure 4). M. complanata (bladed morphotype) typically resides in the surf zone while M. alcicornis (branched morphotype) and the intermediate morphotype reside 1-5 m from the surface [29]. M. complanata (N=43) were split 58% (N=25) clade A dominant and 42% (N=18) clade B dominant. M. alcicornis (N=30) were 87% (N=26) clade A dominant and 13% (N=4) clade B dominant. The intermediate morphotypes (N=9) were 89% (N=8) clade A dominant and 11% (N=1) Symbiodinium B dominant.
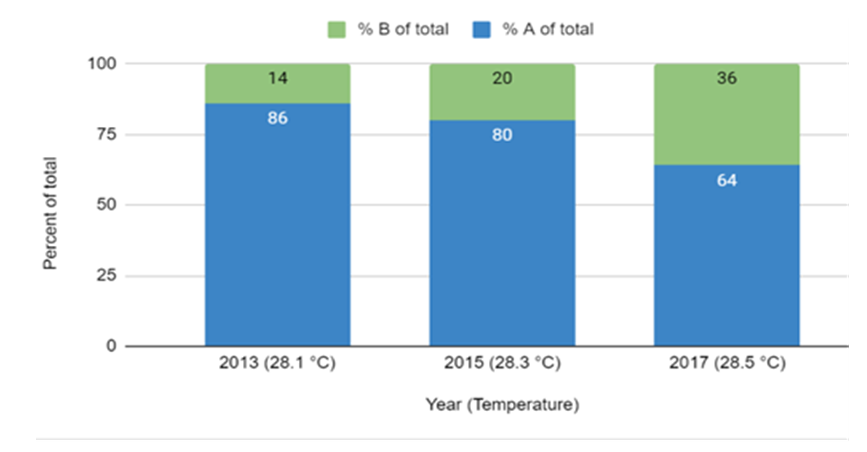
3.3. Year vs Temperature
- The yearly dominance of Symbiodinium clades in Belize Millepores was compared to year collected and temperature (Figure 5). The average SST for 2013 in South Water Cay, Belize was 28.2°C with a total of 35 days above 30°C [38]. A total of 25 Millepore colonies were analyzed, 76% (N=19) were Symbiodinium clade A dominant and 42% of the clade A dominant corals had a clade B background. Of the 24% clade B dominant corals, all had clade A background. Hughes et al. [7] reported that between 2015–2016 record temperatures triggered a global pan-tropical episode of coral bleaching. The average SST in 2015 was 28.3°C with a total of 71 days above 30° [38]. A total of 11 Millepore colonies were analyzed, 73% (N=8) were Symbiodinium clade A dominant and 87% of the clade A dominant corals had a clade B background. Of the 13% clade B dominant corals, all had clade A background. In 2017, Symbiodinium clade A dominance in Belize Millepores had decrease to 56% (N=33) of the 59 colonies analyzed and 91% of the clade A dominant corals had a clade B background. Of the 44% clade B dominant coral, 84% had clade A background. However, the average SST in 2017 was 28.5°C with 118 days above 30°C and 76 of those days were consecutive [38].
3.4. Deep Samples
- An additional survey was conducted on Belize M. alcicornis colonies collected from reefs 5-24 meters deep. A total of 35 colonies were examined; 80% (N=28) were Symbiodinium clade B dominant and 20% (N=7) were Symbiodinium clade A dominant.The background clades for the deep samples were also examined. Deep Symbiodinium clade B dominant colonies contained background clades in 89% (N=25) of the colonies; of those, 96% (N=24) contained clade A as a background clade either alone or in combination with clade C. All the deep Symbiodinium clade A dominant colonies contained clade B as a background clade either alone or in combination with clade C.
4. Discussion
- Changes in the abundance of symbiont populations may provide a mechanism through which coral hosts can better cope with changing environmental conditions. Different symbionts confer different physiological advantages to the host [39,40]. Berkelmans and van Oppen [20] showed that Acropora millepora (branching stony coral) could increase their thermal tolerance by 1-1.5°C by changing their symbiont population from Symbiodinium clade C dominant to Symbiodinium clade D dominant. Baker et al. [23] found that Pocillopora colonies had altered their symbiont associations after the 1997-1998 El Niño Southern Oscillation (ENSO) event to a more thermally tolerant pairing with Symbiodinium clade D, and concluded that it was possible for coral to revert back to their original pairings given enough time without additional thermal stress events. These studies provide support for the Adaptive Bleaching Hypothesis (ABH), which proposes that thermal stress events allow hosts to shuffle or switch their symbiont populations to adapt to environmental change [16,41].The key result of this study is the difference in Symbiodinium clade dominance between San Salvador, The Bahamas, with a yearly SST range of 22-28°C [32] and South Water Cay, Belize, with a yearly sea surface temperature (SST) range of 26-31°C [33]. In 2017, South Water Cay had a total of 118 days of SSTs above 30°C, of which 76 were consecutive [42]. The Millepores in The Bahamas are all Symbiodinium clade B dominant, and the Millepores in Belize are 72% Symbiodinium clade A dominant and 28% Symbiodinium clade B dominant. Of the Millepore samples examined, the only colonies that showed signs of bleaching were Belize Symbiodinium clade B dominant (N=5) (Figure 3). No Belize clade A dominant colonies or Bahamian clade B dominant colonies examined showed signs of bleaching. The difference in dominant symbiont population between the two sites may be due to the temperature differences between the two geographic locations and suggests that clade A may provide Millepores in Belize with a mechanism to cope with the higher temperatures and irradiance they experience. Other researchers have observed similar patterns of changes in symbiont populations in the Caribbean. Venn et al. [43] found that, in Condylactis gigantea (ball anemone), Symbiodinium clade A predominates at inshore and near shore sites that are more thermally variable (15-32°C) and that clade B is the dominant clade at offshore sites with more uniform temperatures (18-28°C). They also observed that anemones that form dominant clade symbioses with clade B are more likely to bleach at SST above 32°C, but anemones that associate with clade A as their dominant clade did not show signs of bleaching. Reynolds et al. [44] used serial irradiation pulse (SIP) fluorescence analysis to determine that Symbiodinium clade A exhibits enhanced capabilities for alternative photosynthetic electron-transport pathways and the ability to undergo pronounced light-induced dissociation of antenna complexes from photosystem II reaction centers. They concluded that these facets of Symbiodinium clade A allow them to promote the survival of most cnidarian hosts under thermal stress conditions and provide the host with resistance to bleaching. Kemp et al. [45] examined Orbicella faveolata (mountainous star coral) before, during, and after a bleaching event in Puerto Morelos, Mexico and found that colonies that contain Symbiodinium clade A3 as their dominant clade prior to a thermal stress event were more resistant to bleaching than colonies that contain clade B17 or C7 as their dominant clades. They concluded that this difference is due to clade A3 maintaining a higher quantum yield of photosystem II than clade B17 or C7.The observations of Venn et al. [43], Reynolds et al. [44], and Kemp et al. [45] are in line with our study which shows an increase in Symbiodinium clade A dominance at more thermally stressed sites, as well as the possibility of an increased likelihood of bleaching in Belize clade B dominant Millepores. The higher thermal tolerance of Symbiodinium clade A appears to allow Millepores to cope more effectively with higher temperatures and irradiance in Belize. Phenotypic plasticity of the Millepores makes morphological characteristics problematic as a tool in taxonomic identification of Millepores [46]. Tepper et al. [29] showed that M. alcicornis and M. complanata are phenotypically plastic and may, in fact, form a single species-complex that cannot be distinguished by morphological characteristics. Although Symbiodinium clade A dominance appears to be significantly more prevalent in M. alcicornis (87%) and intermediate growth forms (89%) than clade B dominance (Figure 4), no definitive interpretations of this difference can be made because of the role that the environment plays in phenotypic plasticity of Millepora [27,28,29,30,31].A single Millepore colony is able to host multiple clades of Symbiodinium at the same time, which provide Millepores with the capability to alter their symbiont populations to cope with changing environmental conditions. In The Bahamas, all the colonies examined were Symbiodinium clade B dominant and 64% of all the colonies examined contained Symbiodinium clade A as a background clade. In Belize, Symbiodinium clade B was found in 75% of all clade A dominant Millepores, and Symbiodinium clade A was found in 87% of all the clade B dominant Millepore colonies. LaJeunesse [37] and Baker [11] noted that although host-symbiont associations are specific, there is flexibility within those specific associations. Fay and Weber [48] found that mixed infection (symbiosis with multiple clades of Symbiodinium) occurs in over 30 genera of scleractinian corals. Symbiodinium clade A’s prevalence as a background clade in Symbiodinium clade B dominant Millepores, both in Belize and The Bahamas, suggests that corals have the ability to shuffle the more thermally tolerant clade A into a dominant position, should the warming trend (0.24°C per decade) in the Caribbean continue and environmental conditions necessitate it [26]. The prevalence of Symbiodinium clade B as a background clade in clade A dominant Belize Millepores suggests that it is possible that the current clade A dominant Millepores were clade B dominant and have shuffled clade A into a dominant position to cope with the higher temperatures they experienced. Additionally, these clade A dominant colonies have the capability to shuffle back to clade B dominance if the temperature stress is alleviated. Although clade C, as a background clade, is almost always the least abundant of symbiont clades present in Millepores, it is possible its presence is an advantage for its host. Baker et al. [49] showed that clade C has a higher rate of nitrogen acquisition and an enhanced ability to confer photosynthetic products to the host.We also examined how symbiont populations fluctuated over time and with different SSTs in Belize. A pan-tropical bleaching event occurred during our study from 2015 to 2016 [7]. Leading up to the bleaching event, in 2013 and 2015, the Millepores were 76% and 73% clade A dominant respectively (Figure 5). However, although the SST rose slightly in 2017, this year could have still been a period of recovery for the Millepores which led to the decrease in clade A dominance (56%). High percentages of coral colonies with clade B backgrounds, may account for this shuffle in dominance. Thornhill et al. [47] observed a similar symbiont population change in Montastrea annularis (boulder star coral) and M. franksi (boulder star coral) from the Florida Keys, which they attributed to recovery of the corals from the thermal stress of the 1997-1998 ENSO event. The results of our study could suggest that the prevalence of Symbiodinium clade A dominance in Millepores is higher before and during a bleaching event and decreases after the event. This change in symbiont population could help the host cope with increased thermal stress during the bleaching event and then help the host start to recover following the bleaching event. Alternatively, the decrease in clade A dominance could also have been a result of a larger sample size in 2017 compared to both 2013 and 2015 collections.Belize Millepore samples collected from deeper reefs (5-24 m) predominantly contain B as their dominant clade. Generally, temperature and irradiance decrease with depth [50]. Therefore, Millepores that reside on deeper reefs may experience less thermal stress and lower irradiance levels. Deep Belize Millepores examined in this study were 80% Symbiodinium clade B dominant, which is in direct contrast to the shallow Belize Millepores that were 72% Symbiodinium clade A dominant. Symbiodinium clade B dominance in Millepores from deeper reefs may be because these colonies do not require the thermal tolerance conferred by Symbiodinium clade A. Finney et al. [51] examined the symbiont populations of six orders of Cnidaria from Barbados and Belize, and found that habitat depth influenced the population of symbionts. In their examination of M. alcicornis and M. complanata at shallow depths from Belize, they found both Symbiodinium clade A4a and Symbiodinium clade B. However, at depths below 5 m with lower ambient light, they no longer found Symbiodinium clade A in the symbiont populations of the Millepores. This is a similar trend to the one observed in the current study, and indicates that Symbiodinium clade A dominant colonies may be less prevalent on deeper reefs because Millepores may be under less thermal stress at deeper depths.
5. Conclusions
- Recent estimates of the impact of climate change predict a 70-90% decline in coral reefs by 2050 [2], which would be devastating for the biodiversity they support and the communities that depend on coral reefs. During the 20th century, SST have risen by 0.74°C and are projected to increase by as much as 4°C during this century [6]. Therefore, understanding the effects of elevated temperatures on Symbiodinium-Millepore symbioses is of the utmost importance. Our study demonstrates that Millepores in warmer waters with more irradiance have different symbiont populations from their counterparts that reside on reefs with cooler temperatures and less irradiance. The presence of background clades indicates that Millepores in both Belize and The Bahamas have the capability to shuffle their symbiont populations to cope with climate change, and that the Symbiodinium clade A dominant Belize Millepores may have already shuffled their symbiont populations to deal with the higher thermal stress they experience in the warmer SST of Belize.
ACKNOWLEDGEMENTS
- This work was conducted in The Bahamas under a permit granted by the The Bahamas Environment, Science, and Technology (BEST) Commission and in Belize under a permit granted by the Belize Fisheries Department to CST. We would like to thank Dr. Troy Dexter, Executive Director of the Gerace Research Centre, San Salvador, Bahamas and Barbara Kelt, Manager of International Zoological Expedition (IZE), South Water Cay, Belize. We would also like to thank Dr. Barbara Christie-Pope for her assistance in completing this research. Completion of this work was made possible by Cornell College Faculty Development Grants (CST) and Iowa College Foundation/R.J. McElroy Student/Faculty Research Program (CST).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML