-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Marine Science
p-ISSN: 2163-2421 e-ISSN: 2163-243X
2015; 5(1): 6-10
doi:10.5923/j.ms.20150501.02
Size at First Maturity of Cuttlefish, Sepia latimanus, from North Sulawesi Waters, Indonesia
Silvester B. Pratasik1, 2, Marsoedi3, D. Arfiati3, D. Setyohadi3
1Postgraduate student of Faculty of Fisheries and Marine Science, Brawijaya University, Malang, East Java
2Faculty of Fisheries and Marine Science, Sam Ratulangi Manado, North Sulawesi
3Faculty of Fisheries and Marine Science, Brawijaya University Malang, East Java
Correspondence to: Silvester B. Pratasik, Postgraduate student of Faculty of Fisheries and Marine Science, Brawijaya University, Malang, East Java.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Biological overfishing could occur from either excessive and immature individual exploitation or habitat destruction. This study was aimed to estimate size at first maturity of cuttlefish, Sepialatimanus, collected from North Sulawesi waters. All samples were measured and observed their maturity level. Based on these data, the dorsal mantle length (DML) at first maturity was assessed for minimum legal size determination. Results showed that the cuttlefish samples had maturity level range from immature to post-spawning conditions, while the size at first maturity was estimated as 16 cm DML. Maximum DML was estimated as 55.53 cm and growth coefficient as 0.248. The deviation of mean DML from maximum dorsal length was also considered to see the population condition.
Keywords: Cuttlefish, Sepialatimanus, Size at first maturity
Cite this paper: Silvester B. Pratasik, Marsoedi, D. Arfiati, D. Setyohadi, Size at First Maturity of Cuttlefish, Sepia latimanus, from North Sulawesi Waters, Indonesia, Marine Science, Vol. 5 No. 1, 2015, pp. 6-10. doi: 10.5923/j.ms.20150501.02.
1. Introduction
- Cuttlefish are one of the high economic value exported fisheries resources, and therefore, they are highly exploited in the world. Nevertheless, they are categorized as high risk species, due to their high uncertainty of being caught before spawning and low productivity [1]. Cuttlefish, Sepia pharaoni (Stage IV) in Persian Gulf has 53 ova in a 198 mm female to 1,589 ova in a 254 mm female [2] and common cuttlefish, S officinalis, has a number of 3,700-8,000 ova [3]. Increasing demand of cephalopod meat has made this resource be more and more hunted through increased number of fishing operations and fishing gear development. In English channel and Japan, S. officinalis and S. esculanta, are caught using trawl, set net, gill net, trammel net and spawning-substrated trap [4-7] in the spawning areas of the cuttlefish. Extensive coastal development, environmental destructions of the bay area, excessive exploitation, and competition with other fisheries have caused cuttlefish catch decline [4].Sustainable use of fisheries resources while avoiding overexploitation is to maintain the equilibrium between the amount of fishing and the conservation. Overfishing can occur when too many small individuals are caught or no enough time is left to small individuals to grow (growth overfishing), young individuals entering the fishing ground are fished (recruitment overfishing), and the effort is higher than that maximizing the economic rent (economic overfishing) [8]. Size at first maturity (lm) or 50% of mature individuals has been taken as reference point of minimum legal size to prevent stock depletion. It has been used by many fisheries managers as management measure of fish stock [9-11] over 100 years to let mature individuals spawn at least once or to protect immature individuals [12]. Overall considerations should be based on ecosystem approach, such as impact on stock, habitat, food web and non-target species and non-target species [13, 14], beside productivity and mortality of the species, predator–prey interactions, competition, carrying capacity, and population variability, and environmental parameters [15, 16].One of the shallow water cuttlefish is Sepia latimanus. This species migrates inshore for spawning and laying eggs in coral reef ecosystem. According to FAO [17], Sepia latimanus is the second biggest cuttlefish after S. apama with maximum dorsal mantle length of 50 cm and total weight of 10 kg. Froese [18] emphasized the relationship between fisheries management and life history theory that size composition of catches should be applied from 3 simple ideas: a) catch composition represents mature individuals (Pmat); b) size composition of the catch represents the highest catch of a cohort (Popt); and c) catch composition reflects the conservation of big-sized mature individuals. In Indonesia, there is no cephalopod fishery, and all catches are obtained as an incidental catch of traditional fishermen, and therefore the catch number is low, beside no size limit regulation is applied. In North Sulawesi, cuttlefish, S. latimanus, are mostly caught by speargunners in shallow waters by taking advantage of their migration to the coral reef area for laying eggs or jig fishing. Many parts along the coast were also developed for commercial areas causing extensive area of coral damages, beside destructions from other anthropogenic activities that will affect the presence of potential cuttlefish spawning grounds. There is also no cuttlefish study, but FAO report [17], conducted in Indonesia waters, particularly in North Sulawesi waters. This study was intended to estimate the size at first maturity of S. latimanus from North Sulawesi waters, then use it as a basis of minimum legal size determination. The study outcome is expected to be able to increase the understanding on conservation in relation with size limit implementation in cuttlefish population.
2. Method
- This study used a survey method. Samples were collected from Arakan, Minahasa regency, Manado, Likupang, and Bitung, North Sulawesi (Fig. 1), as fishermen’s catch from April to November 2014. Cuttlefish were mostly collected from local fishermen and traditional market. Their fishing techniques were also recorded through interviews with local fishermen. Samples were measured the dorsal mantle length (DML). Maturity level was assessed through dissection and looking at the egg condition in the body cavity [19].
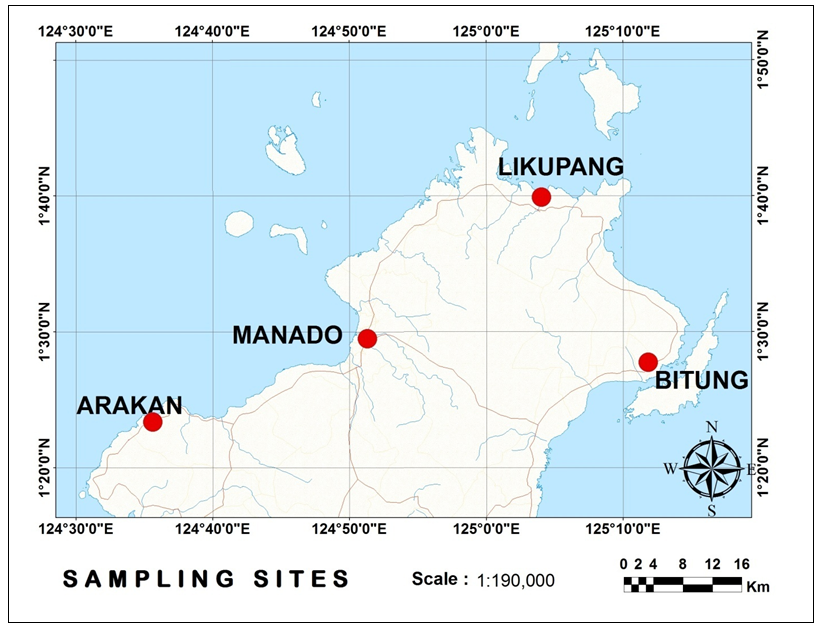 | Figure 1. Study site |
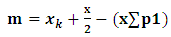 | (1) |
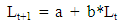 | (2) |
3. Results and Discussion
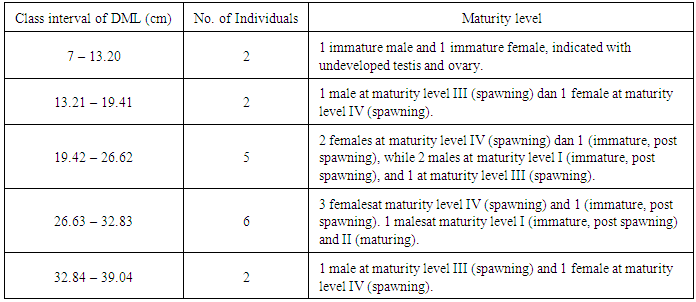
- During the study, 17 cuttlefish, Sepia latimanus, collected from fishermen and intermediate fish sellers from Arakan to Bitung were for size at first maturity analysis. It resulted from the difficulty to obtain sufficient number of samples. All catches were gained from traditional fishermen using traditional speargun at the depth of about 10 m or jig fishing. Other previous cuttlefish studies [3, 2, 22] could collect more sufficient number of samples due to the use of more efficient fishing gear, such as trawl, and reaching the cuttlefish habitats in deeper waters. This study was based upon traditional fishermen’s catches using simple fishing gear. Therefore, the catches could be incidental and in small numbers, because the speargunners did not hunt for specific fish target, but various marine resources that could be caught by the gear, including the cuttlefish. The use of this fishing gear is also generally done around the coral reefs. S. latimanus is usually caught when they migrate to the coral reef area for laying eggs. During the study, at least 8 jig fishing activities were conducted together with local fisherman but no cuttlefish were caught. Similar condition was found for jig fishermen (pers. com.) that Sepia latimanus was rarely caught in jig fishing, and the catches were large-sized squids. According to FAO [17], cuttlefish, Sepiidae, are highly important fisheries resources to exploit and usually obtained as bycatch of bottom trawl fisheries. In artisanal fisheries, with good knowledge of their biology and behavior, cuttlefish were caught using various selective fishing gears, including fish aggregating device (FAD), such as light and subtrate for laying eggs, and various fishing gears, such as speargun, trammel net, hoop net, hand line, jig, baited pot, and etc. In North Sulawesi, cuttlefish are fished with speargun and jig. Netting is only used to catch small squids, Loligo sp., in certain season, and some fishermen use scoop nets at night under a low light intensity. S. latimanus belongs to insufficient data fisheries resources category [23], and even endangered species even though they have very wide geographic distribution [17]. It is also demonstrated by present field study in North Sulawesi waters. Therefore, our first research plan established around Manado Bay had to be widened westwards to Arakan waters, Minahasa regency, and eastwards to Lembeh strait, Bitung, because of difficulty in sample collection. Coastal development could be one of the causes for low fishermen’s cuttlefish catches in this area, reflecting environmental disturbance and possible potential habitat destruction. Coastal areas of North Sulawesi waters have recently been developed for commercial area development, and extensive areas of coral reef ecosystem are reclaimed and sacrificed. All these development activities have caused environmental damages and disturbances, particularly potential nursery grounds in coastal ecosystems. High decline in cuttlefish catches in Japan was also reported from environmental damages in bays, excessive exploitation, and competition with other fisheries [4], so that Japanese fishermen incubated the eggs attached on the traps and developed spawning areas for mating and protected the cuttlefish resources [24]. In English channel, the coastal spawning areas were also controlled to prevent population depletion before the cuttlefish have chances to spawn that sufficient number of cuttlefish was left to be mature and to ensure that spawning ground conditions did not disturb the egg development. It includes leaving the eggs attached to the cuttlefish traps in the protected marine areas to enable the eggs to hatch [5]. This action was to take advantages of the cuttlefish behavior to attach their eggs on seaweeds, sessile animals, artificial structures and sea bottom [5, 6]. In North Sulawesi waters, S. latimanus used certain branching corals to lay their eggs one by one (pers. obs) reflecting that coral reefs plays very important roles on providing shelters for the eggs and possibly the youngsters, but it is also a potential fishing area for speargunners that could restrain the egg laying activities of the cuttlefish.Based on sample collection, cuttlefish obtained in this study represented both young and mature individuals with size range from 7 cm to 34 cm DML that was separated into 5 class intervals (Table 1). Only 2 individuals were immature small cuttlefish, while the rests had maturity level at spawning and post-spawning conditions meaning that they belonged to adult individuals. It could result from that coral reefs was used for spawning and egg laying activities, and thus, only large and sexually mature individuals visited the area. Small individuals were not observed in the dive survey indicating that small individuals may occupy different habitats in deeper waters. This fact is in line with FAO’s report [17] that this species disperses up to 30 m water depth which also explains the low number of individuals obtained in this study, and therefore, more effective sampling equipment is needed to reach the deeper habitats where there is possibly high abundance of S. latimanus. However, since this study was the first cuttlefish study in North Sulawesi waters, and no information is available on which depth this species is abundantly distributed, the jig fishing was done based on information from local fishermen who used to fish cuttlefish at around 15-20 m water depth.
|
ACKNOWLEDGEMENTS
- We are highly grateful to the Government of Indonesia through the Directorate General of Higher Education for the financial support of the study. We also thank to Yayasan Minahasa Raya for financial assistance to this study. Finally, we would greatly appreciate DR. Unstein Rembet who put few times for discussion in order for this paper completion as well.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML