-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Marine Science
p-ISSN: 2163-2421 e-ISSN: 2163-243X
2014; 4(2): 44-48
doi:10.5923/j.ms.20140402.03
Effect of a Ballast Water Treatment System on Survivorship of Natural Populations of Marine Plankton in Persian Gulf, Iran
Maryam Shapoori1, Mansoure Gholami2
1Department of Fishery, College of Natural resources, Savadkooh Branch, Islamic Azad University, Savadkooh, Iran
2Department of Fishery, College of Natural resources, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran
Correspondence to: Maryam Shapoori, Department of Fishery, College of Natural resources, Savadkooh Branch, Islamic Azad University, Savadkooh, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
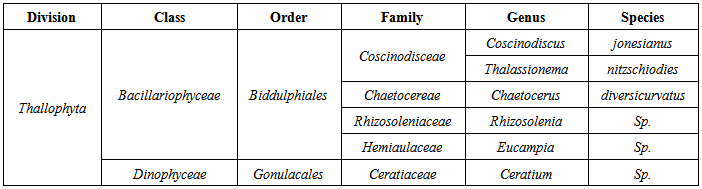
We investigated the viability of phytoplankton from ballast water of commercial and petroleum ships berthed at the ports of Kharg Island, Iran. Ballast water was carried in regional seas in order to assess the efficiency of this type of ballast water management at reducing the abundance and diversity of phytoplankton. Although there was an overall reduction in the average abundance and number of taxa after exchange this was not consistent between tanks and voyages. On some occasions there were changes in species composition after exchange. Mentioned research was a part of Regional and National plan and protection for environmental impact assessment and also control and prevention of navigation activities. The result showed that species were dominant in the samples of Ceratium sp., Rhizosolenia sp., Coscinodiscus jonesianus, Eucampia sp., Chaetocerus diversicurvatus Thalassionema nitzschoides. Factors such as the depth of the water during the exchange process, the season and the method of exchange influenced the efficacy of the exchange process. The variability in the results after exchange mean that this is unlikely to be a ballast water management method that would give consistent results and careful consideration would have to be given to the suitability of using this method in regional seas as a means of reducing the risk of introducing non-native species.
Keywords: Ballast water, Phytoplankton, Persian Gulf, Iran
Cite this paper: Maryam Shapoori, Mansoure Gholami, Effect of a Ballast Water Treatment System on Survivorship of Natural Populations of Marine Plankton in Persian Gulf, Iran, Marine Science, Vol. 4 No. 2, 2014, pp. 44-48. doi: 10.5923/j.ms.20140402.03.
1. Introduction
- Ship ballast water is widely considered to be the most influential vector for transporting organisms outside of their bioregions, which has been found to be responsible for human-mediated disturbance to species distributions and change in community structures [1-3].Ballast water can include many organisms from diverse taxonomic groups originating from various bio-geographic regions [1, 4-8]. Successful invasion is facilitated by a multiple-step process in which organisms taken up in ballast water tanks first survive during voyages and then must be sufficiently abundant to reproduce in the receiving waters [9]. Following introduction, the successful establishment of foreign organisms has been linked with abiotic and biotic characteristics of the recipient community as well as characteristics of the invading species [7]. Genetic variability, body size, physiological tolerance, and reproductive strategy can play a role in promoting invasions of non-indigenous species (NIS). Furthermore, although not invasive traits, relatively large populations and broad distributions of NIS will increase their chances for establishment. Until recently, most ballast water studies have focused on the examination of species found in the ballast tanks at the beginning, middle, and end of voyages [2, 10-11]. Knowledge of species composition and abundance in ballast water tanks can provide information about ways in which species have interacted during the cruise period. However, this is not sufficient to indicate adaptive ecology and growth strategies of foreign species after their introduction to receiving waters. Organisms surviving the harsh environment of ballast tanks may face environmentally unfamiliar recipient waters after being discharged. The success of invasion is most likely dependent upon characteristics of the receiving waters, which are classified as stressors or facilitators [9]. In phytoplankton, reproductive performance is facilitated by growth, which is affected by environmental factors such as temperature, salinity, inorganic nutrients, and light intensity [12-13]. Given that environmental similarity between ports is relatively important, the success of invasion will be increased when species are introduced into port waters that fall within their preferred environmental range [14]. Temperature has a strong influence on marine invasions, because it prevents some exotics from becoming established at release sites where water temperatures are cold relative to the invaders’ normal temperature range [10, 15]. The salinity of receiving water is also an important limiting factor for aquatic species [16], although estuarine dinoflagellates are less sensitive to salinity [15]. Across oceanic barriers, the successful transfer of exotics has been intensified by coastal eutrophication and by the size and volume of vessels [15, 17], and by favorable local environment [18-19]. The recognition of ballast water as a probable vector for organisms with potentially negative impacts on ecosystems prompted numerous countries to find methods for reducing potential risks from invasive species. As foreign species successfully pass through phases from introduction to establishment, the community structure and function of coastal ecosystems in receiving waters will be directly or indirectly vulnerable to threats such as toxic phytoplankton blooms. Thus, the viability of introduced species in recipient waters can provide important information for categorizing the potential threats from foreign species found in international commercial ships entering ports.The Persian Gulf is a unique environment. It is a semi-enclosed marginal sea about 1000 km long, about 200-300 km wide, with a mean depth of 35 m and has a total of 6000 km3 volume. North of Shatt Al-Arab estuary it is bound by land and to the south connected to the Gulf of Oman through the narrow Straits of Hormuz that lead to the Persian Sea (Figure 1). Estimates of freshwater inflow into the Gulf range was between 5106 to 100106 m3 [20]. The rivers Tigris and Euphrates in the north discharge annually each 45.3106m3 water, and 57.6106 and 4.8l06t sediment, respectively [21]. In the present study, the responses of phytoplankton in ballast water to conditions simulating possible abiotic conditions in recipient waters were examined to understand environmental factors affecting the survival success of introduced species.
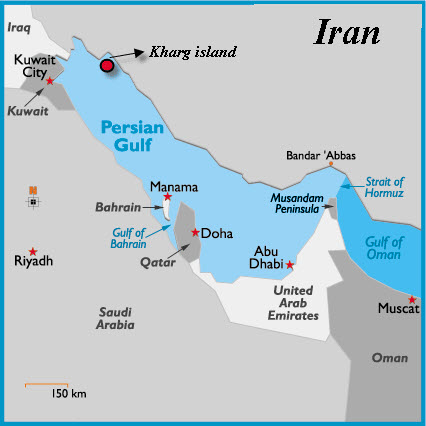 | Figure 1. Map of Kharg in Persian Gulf |
2. Method and Materials
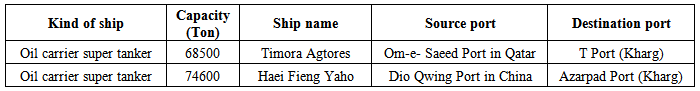
- This study focused on oil ships at terminal in Persian Gulf. Biological and environmental conditions were recorded from samples obtained from the ballast water tanks of T port in the east and Azar pad port in the west south of island, to investigate the survival success of phytoplankton in ballast water after discharge into the coastal waters of Kharg (Figure 2). Water samples were collected for phytoplankton identification basis from 2 locations. Phytoplankton samples were collected by towing a 67 lm meshes net, to concentrate the plankton into a 50 mL sample, which was then preserved with Glutaraldehyde (final 5%). Phytoplanktons were identified to the genus level using light microscopy with the assistance of the identification guide by Tomas [22]. A Motic AE31 inverted microscope with Leica EC3 camera used in conjunction with the Leica Application Suite (LAS) EZ V1.6.0 digital photography software was used to aid the identification of the phytoplankton. The ballasting sites and the names of available vessels are shown in Table 1.
|
 | Figure 2. Location of the vessel during the exchange process (T port and Azarpad Island inKharg Island) |
3. Result
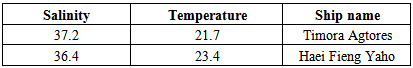
- In this study, salinity and temperature parameters were measured. Most salinity was for Timora Agtores, and most temperature was for Iran Atrak. The least salinity was for Haei Fieng Yaho (Table 2).
|
|
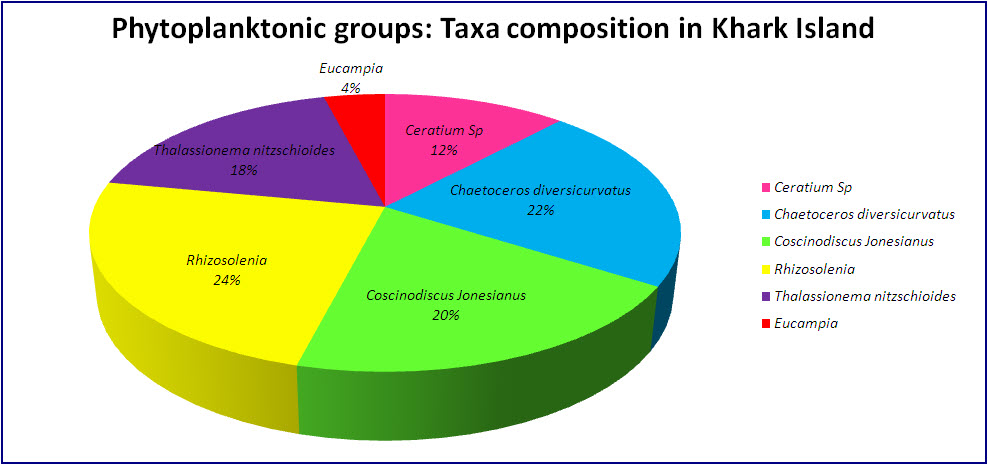 | Figure 3. Percent composition of various phytoplankton in Kharg Port (Persian Gulf) |
4. Discussion
- Many ballast studies have consisted of completion of checklists of preserved samples collected from ballast water after and before ballast water exchange or at the end of voyages [2, 10, 23-24]. It is very difficult to determine the viability of phytoplankton from microscopic observations of preserved samples, and viable resting stages frequently could be overlooked. However, little is known about the viability of phytoplankton in initial samples when incubated or about environmental factors affecting the colonization of phytoplankton in ballast water in new environments. After plankton in the ballast tank are discharged into shipside waters, physical conditions, voyage duration, and taxonomic and ontogenetic differences influence their survival [11]. Hydrographic similarities between ballast tank water and receiving waters could be prerequisite for the successful establishment of phytoplankton, which must then persist in sufficient concentrations to reproduce. In this study, the diversity of species found included diatoms and dinoflagellates. Phytoplankton (dinoflagellates and diatoms) could increase very quickly through asexual reproduction under the proper light, nutrient, and temperature conditions. Irradiance and temperature have been identified as two important environmental factors regulating the intrinsic growth of phytoplankton. Nutrient availability and higher temperatures also enhanced the growth rate of diatoms [25]. This study showed a decrease in phytoplankton abundance and number of geniuses. These decreases in nutrient and temperature conditions in receiving waters became optimal for introduced phytoplankton; the potential for their survival would become intensified in the waters of Kharg Island and Bandar-Abbass. This ballast water sampling study was first carried out in Persian Gulf. Short voyages may transfer greater abundance of live plankton than do voyage of longer duration. The hypothesis that longer sea voyage results in fewer phototrophic species being observed at the end of the voyage were supported by Chu et al [26].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML