-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Marine Science
p-ISSN: 2163-2421 e-ISSN: 2163-243X
2014; 4(1): 21-25
doi:10.5923/j.ms.20140401.03
Investigating the Effect of 17α-Methyl Testosterone Hormone on Hue and Color Measurement of Scatophagus Argus
Mojtaba Shahidian1, Shahla Jamili2, Faeze Rezaiee1, Niusha Amri3
1Department of Marine Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Iranian Fisheries Research Organization, Tehran, Iran
3Science and Research Branch of Islamic Azad University, Tehran, Iran
Correspondence to: Mojtaba Shahidian, Department of Marine Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
17α-methyl testosterone is an androgen plays role in sexual cycle and affects increasing growth and changing secondary attributes. In the present study, the effect of three different doses of this hormone (300, 400 and 1000 mg/kg/kg) on secondary sexual characteristics (Hue and value) of Scatophagus argus was investigated. The results of this research indicated that there is a strong relationship between the physiological condition of fish with the skin color and secondary sexual characteristics and by increase of dose, the glitter in the fish’s tint was increased. Tint, the bottom of body in fish samples with doses of 0, 300 and 400 ml/kg had no significant difference (P>0.05), while in a dose of 1000 ml/kg of the allotment it had significant difference (P<0.05) and the maximum tint was observed in samples with 1000 ml dosage. These results showed a tendency to increase red tint, while in all investigated doses, significant differences in the rate of tint between tailfin and body fin (P<0.05). On the other side, the factor of tint in the tailfin of fish had significant difference too (P<0.05) and indicates a decreasing procedure toward red tint.
Keywords: 17α-methyl testosterone, Secondary sexual characteristics, Color measurement, Tint, Scatophagus argus
Cite this paper: Mojtaba Shahidian, Shahla Jamili, Faeze Rezaiee, Niusha Amri, Investigating the Effect of 17α-Methyl Testosterone Hormone on Hue and Color Measurement of Scatophagus Argus, Marine Science, Vol. 4 No. 1, 2014, pp. 21-25. doi: 10.5923/j.ms.20140401.03.
1. Introduction
- Scatophagus argus belongs to the scatophagidae family from the vernacular fish of the Persian Gulf. These fish are diadromous, they naturally live in seawater, but they can easily enter fresh waters. We can mention to its small head and couple short, but dynamic fins in its anatomy[12]. The color pattern on the body of this fish is changed by feeding on different nutrients and it is included of green, violet, black, yellow, brown and white in the ventral part. If its habit of feeding known as a carnivore, violet and brown colors would be more revealing and if it’s become an herbivore, green and yellow colors are revealed. This fish has a white belly. In female fish, this white stain is only in lower fins, however, in the male fish, it starts from the beneath of its gills and continues to its anus. They usually spawn in brackish water and in its genesis in freshwater, eggs hardly become larvae. The body of this fish shows the maximum coloring in relatively saltwaters[1]. The attractiveness and behavior of male fish are effective in being selected by the females, such behavior is observed in some other fish too. For example, in Guppy, the color of males, affects selection of females[6; 10; 11, 21]. During the couple selections, prior to mating, the females prefer males with relatively high attractive colors[20], especially the carotenoid color (orange, red and yellow)[7, 8]. Many of these specifications are considered as secondary sexual characteristics and are under the influence of the rate of blood hormones. It is specified that the color of a fish’s body[14; 17) and development of Gonopodium in young males[9; 17] are controlled by Gonad androgens. Bayley et al.[3] Studied the rate of orange color proportioned to the body surface as a secondary characteristic under influence of androgens in Guppy fish[3]. Also in the studies of Nielsen and Baatrup[15] the orange color was investigated as secondary indicators and determining factors in success of mating.
2. Materials and Method
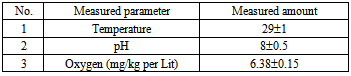
- In order to perform this study, 4 different doses of 17α-methyl testosterone hormone (0, 300, 400 and 1000 mg/kg/kg) with three replications were considered[18]. Each replication was included of 3 samples. The commercial methyl testosterone pills of Abureyhan Pharmaceutics Company containing 25 mg/kg of active ingredient that after calculation of the rate of active ingredient, it has become powder and mixed with their food. The fish were fed by allotment containing hormone for 45 days. In order to feed them, the Biomar food of France with a diameter of 0.8 mm is used twice in a day in saturation level. (The ingredients of biomar food are included of protein: 56%, fat: 18%, Fiber: 0.4%, ash: 10.5%, moisture and other compositions: 15.1%). In order to keep the water quality, half of the water of the aquarium was changed every three days. The aquariums were fully fanned during the test period. easuring qualitative parameters of water were conducted during the test. The general results of measuring qualitative parameters of water are shown in table (1).
|
 | Figure 1. Scatophagus argus |
 | Figure 2. Treatments |
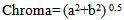 | (1) |
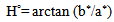 | (2) |
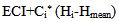 | (3) |
3. Results
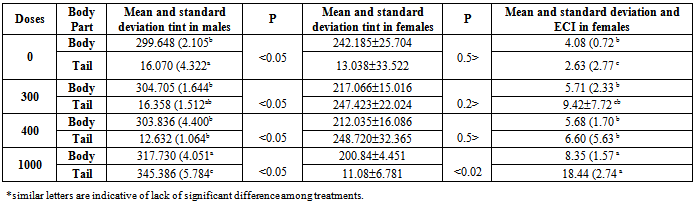
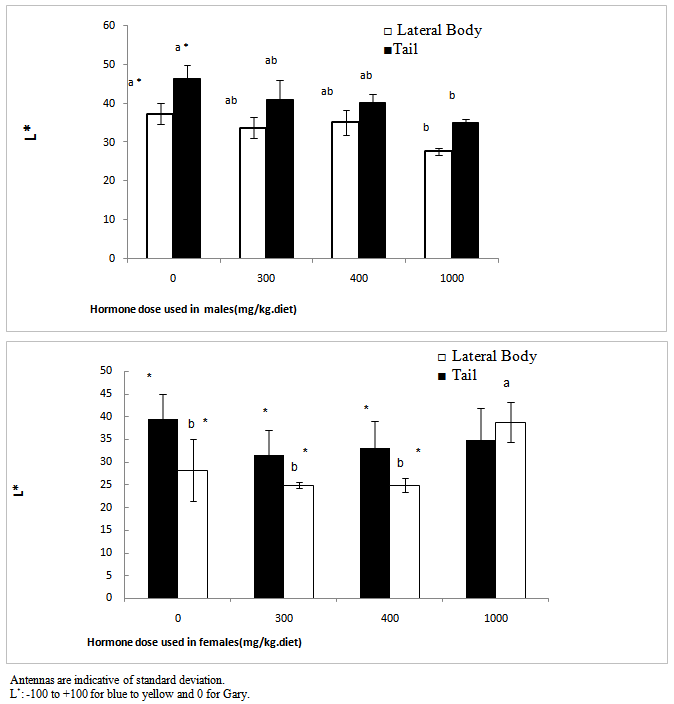
- As it is shown in table (2), by the increase of hormone dosage, significant differences in light rate (L*) of the body and tailfin of the fish were observed. Also, the light rate of the body of fish in 1000 mg/kg dosage had the maximum rate compared to three other doses and didn’t have significant differences in light between different doses at the tail area of the fish (P<0.05). At all hormone doses, the rate of light in tail area has been significantly more than body area (P<0.05).
|
4. Discussion and Conclusions
- In fish investigated in the present study, an interesting similarity was observed in the change procedure for tint and the light rate. In the area of tailfin of the control fish, the highlight was observed, in this dosage fewer tints is toward red and more tint is toward yellow, but in a dosage of 300 mg/kg the light rate decreased suddenly which was due to tendency to red tint. In higher doses also the tendency was toward increase of red tint and in 1000 mg/kg red tint was dominant and in this dosage that in this dosage also indicated an increase in doses 300 and 400, but it was also less than control treatment which can be due to change in melanin, drozopetrin and cartenoeid pigments, but determining its definite factor requires measuring the change process in skin pigments. In the side part of the body, by the increase of tendency from 270 tint (blue) which the light is decreased due to melanin pigments and other pigments, but in 1000 mg/kg, by the increase of the tendency of tint toward red tint, the light would increase too. This issue happened with the decrease of tendency in blue tint and its creating pigments. Color changes due to the effect of hormone was less in the body area in a way that only in 1000 mg/kg dosage it caused significant differences (P<0.05). Pavlidis et al.[18] related the low amount of light factor as a result of high rates of melanin and the pigment percentage of cartenoeid in the skin. The results of other studies also indicated an increase in red color being exposed to methyl testosterone or androgen materials. In male mature Guppy fish, the areas with orange color were influenced after being exposed to high densities of acetyl phenol or estradiol[2: 23]. In the study of Pikulkaew and Wongsathein[19] the intensity of red color in the tailfin indicated a decrease under specific densities of materials similar to Di Chlorodiphenyl-trichloroethane (an estrogen material). Bayley et al. In 2002 concluded on young fish that the fish exposed to anti-androgens of vinclosolin and pl, p, Chlorodiphenyl-trichloroethane (DDT) -DDE and flotamid indicate a reduction in the orange color. Devasurenda et al. (2007) are reporting increases in the rate of drozopetrin that creates red spots in this fish by use of density of 250 mg/kg of food from one analogue androgen and they attributed the creation of orange spots on this fish under the influence of androgens. drozopetrin and cartenoeid play role in the creation of chroma of orange spots, thus the existence of these two pigments can be studied in the fish. The factor of light also refers to the amount of light that reflects the subjective color or passes it through itself and this issue would cause distinction between light and dark colors.The fish can change their color by distributing and gathering pigments in chromatophore quickly (physiological color change) or by changing the rate of pigments or the number of chromatophore (morphological color change)[22]. Vast and slow expansion of pigments and especially the red color in this study during the test period is indicative of this fact that a change of color in studying fish was a morphological type and its reason was changed at the rate of pigments or the rate of chromatophore. Joaki Larsson et al.[13] have concluded the same result about Guppy fish exposed to androgen materials and increases in red color.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML