-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Marine Science
p-ISSN: 2163-2421 e-ISSN: 2163-243X
2013; 3(1): 30-37
doi:10.5923/j.ms.20130301.03
Deep Chlorophyll Maximum in Western Equatorial Atlantic - How does it Interact with Islands Slopes and Seamounts?
Tarcisio A. Cordeiro 1, Frederico P. Brandini 2, Ricardo S. Rosa 1, Roberto Sassi 1
1Department of Systematics and Ecology, Federal University of Paraíba, João Pessoa, 58059-900, Brazil
2Oceanographic Institute, University of São Paulo, São Paulo, 05508-120, Brazil
Correspondence to: Tarcisio A. Cordeiro , Department of Systematics and Ecology, Federal University of Paraíba, João Pessoa, 58059-900, Brazil.
| Email: | 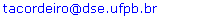 |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This paper investigates the dynamics of the deep chlorophyll maximum layer (DCML) along the Fernando de Noronha Ridge in the oligotrophic western equatorial Atlantic off northeast Brazil. Mixing processes between water masses above and below the thermocline may occur in a scale of tens of meters around the 24.5 to 25.5 isopycnals, where photoau- totrophs are concentrate at the bottom of the euphotic zone. The primary production at the DCML can provide 210 (±170) metric tons of Carbon per day to the benthic community at the Rocas atoll. The behavior of isopycnals in deeper and strongly stratified waters suggest that internal waves may improve primary production, by lifting the deeper and nutrient-rich South Atlantic Central Water upwards and carrying the autotrophs to brighter depths.
Keywords: Deep Chlorophyll Maximum, Rocas Atoll, Northeast Brazil, Western Equatorial Atlantic
Cite this paper: Tarcisio A. Cordeiro , Frederico P. Brandini , Ricardo S. Rosa , Roberto Sassi , Deep Chlorophyll Maximum in Western Equatorial Atlantic - How does it Interact with Islands Slopes and Seamounts?, Marine Science, Vol. 3 No. 1, 2013, pp. 30-37. doi: 10.5923/j.ms.20130301.03.
Article Outline
1. Introduction
- The upper layers of tropical and subtropical ocean basins are physically stratified with a shallow mixed layer over the main pycnocline, along which deep chlorophyll maximum layers are always detected and maintained through the balance between the growth of pico-sized photoautotrophs and losses by grazing[1]. This are usually dominated by picosized flagellates and the phototrophic cyanobacteria Synechoccocus and Prochloroccocus, which have been reported to account for most of the autotrophic biomass in the oceanic basins[2, 3], particularly Prochloroccocus marinus which may account for 50 to 80% of the planktonic primary production in tropical and subtropical oceans, growing well adapted to extremely low light intensities at deeper euphotic layers[4, 1, 5]. The global distribution of the Prochloroccocus species makes them an important step of the oceanic biological pump. However, besides the understanding about the role of the DCML for the oceanic carbon budget it is also necessary to know its spatial dynamics at regional scales. Particularly, how is it affected by the interactions between hydrography and the topographic features like island slopes and seamounts? Does the DCML improve the productivity of the benthic communities? This investigation describes a conspicuous deep chlorophyll maximum layer along the pycnocline over the Fernando de Noronha Ridge (FNR) in the western equatorial Atlantic and discuss some local processes that may affect the local carbon flow.
2. Material and Methods
2.1. Study Area
- The Fernando de Noronha Ridge (FNR) off northeast Brazil is a chain composed by seamounts, one guyot, atoll and islands, aligned in an approximate east-west direction, extending from 32° to 36° W and at 3° to 4º S (Fig.1). For a long time this chain was considered as a branch of the mid Atlantic ridge; nevertheless, more recent authors, ex.[33] and references inside, assumed that the ridge was formed by the passage of the South-American plate over a hotspot. Fernando de Noronha Archipelago and Rocas Atoll are only two peaks of this submarine mountain system, rising 4 km from the ocean floor. Together they make up >50% of the insular South Atlantic coastal area surrounded by clear and warm oligotrophic waters of the South Equatorial Current. The benthic system maintains a significant part of the marine biodiversity for the entire South Atlantic basin and support important breeding and feeding grounds of reef fishes, tuna, shark, turtle, marine mammals and the largest concentration of seabirds in the Western Atlantic[34]. A highly diverse benthic community colonizes the islands slopes[6, 7], in Rocas coralline algae is the main atoll's building frame. Water physical stratification in the upper layers is kept by the convergence of Equatorial Surface Water (ESW) carried into the study area from east by the Atlantic South Equatorial Current (ASEC), and keeping year-round upper layers with water temperatures and salinities of >20°C and >35,5, respectively[8, 9, 10, 11]. Lower salinities at the very surface waters are due to the high precipitation at the intertropical convergence zone[8, 10]. High salinity (up to 37) maximum deep layers are formed at surface through intense evaporation in the region known as the Southern South Equatorial Current (SSEC), which is feed by the Benguela Current westwards drift (8 to 25º S off the African coast), it follows the thermocline in the σ = 24.5 to 25.5 isopycnal range[11]. Low nutrient concentrations in the upper mixed layers keep low rates of primary production with mean integrated chlorophyll concentrations <50 mg.m-2[12, 13] most of which concentrated along the pycnocline. Reference [14] reported that these deep chlorophyll maximum layers are dominated by the picocyanobacteria of the genus Prochlorococcus, growing between 77 and 120 m and being well adapted to extremely low light intensities, about 1 to 0,1%of surface light.
2.2. Field Sampling
 | Figure 1. Position of the oceanographic stations |
3. Results
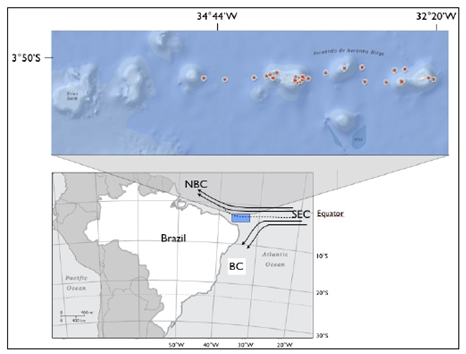
- The T/S diagram for all stations from surface down to 200 meters shows the presence of a strong thermocline in the Equatorial Surface Waters (ESW) with salinities >35,5 and temperatures >20ºC. Below the thermocline the South Atlantic Central Waters (SACW) was present, with temperatures ranging from 11,3 to 20ºC and salinity from 35,5 to 36,4. Some thermohaline pairs with salinity below 35,5 were found at surface of the stations 1, 27 and 2, indicating the occurrence of rain (Fig 2).
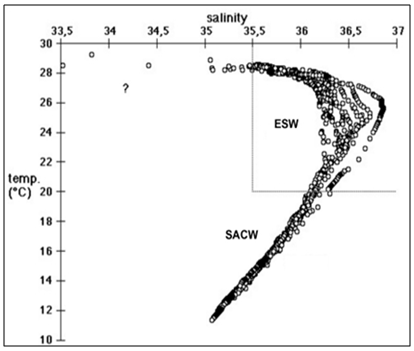 | Figure 2. T-S diagram of 2570 thermohaline pairs |
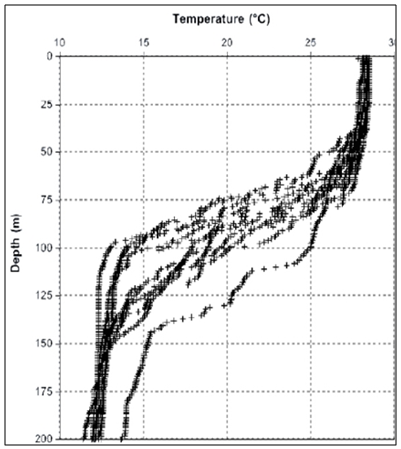 | Figure 3. Vertical profiles of temperature |
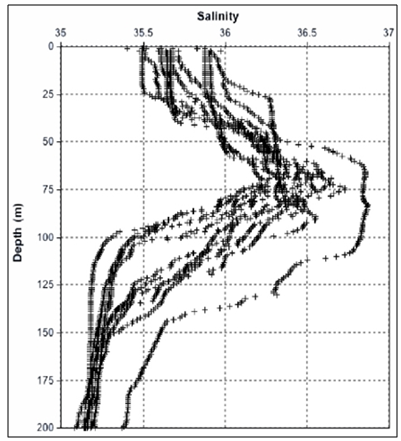 | Figure 4. Vertical profiles of salinity |
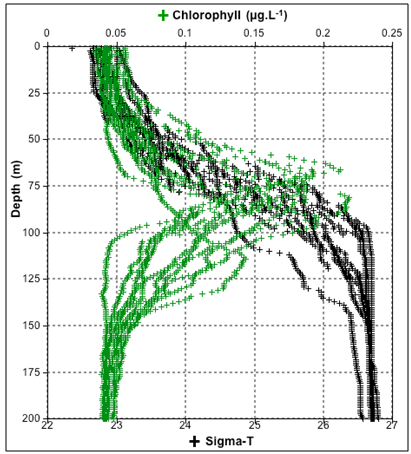 | Figure 5. Vertical profiles of chlorophyll and density (sigma-t) |
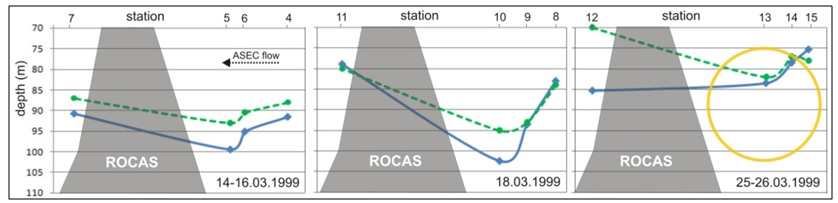
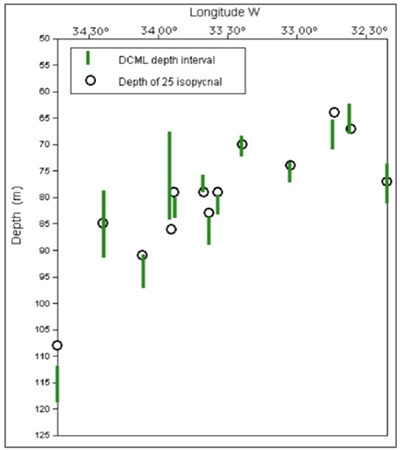 | Figure 6. DCML and 25 isopycnal depths along the transec |
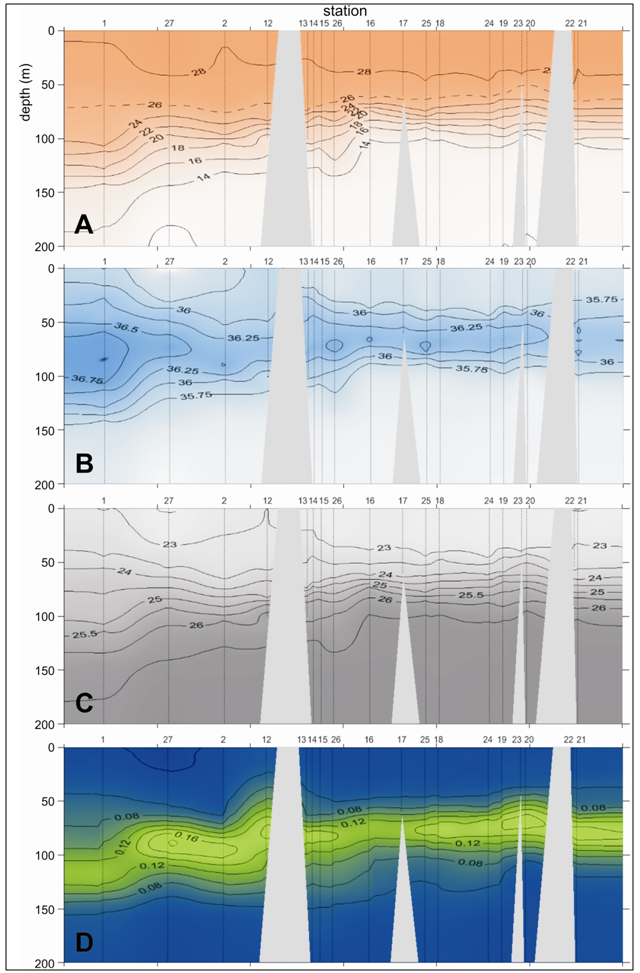 | Figure 7. Isolines of salinity (A), temperature (B), density as σ-t (C) and chlorophyll (D) |
4. Discussion
- The strong stratification of the oligotrophic ESW keeps a shallow nutrient poor mix layer with chlorophyll concentrations as low as in most tropical waters of the oceanic gyres[2]. Mean and integrated chlorophyll down to 200 meters depth varied respectively from 0,05-0,11 µg.L-1 and from 13,0 to 15,6 mg.m-2, what is consistent with previous studies in the same region[12] and in similar hydrographic domains of Brazilian waters[9, 13]. DCML has been reported along pycnoclines off northeast Brazil by[16, 14], the second detected it near our study transect in depths of 0,1 to 1 % of surface light (77-120 m). Two remarkable points in our study is the stretching of isopycnals and the gradual deepening of the DCML westwards. The first may be assigned to the accumulation of ESW against seamount or continental slopes located further west of our sampling transect, shifting the flow of the ASEC northwards to form the North Brazil Current at approximately 35°W[17, 18, 10]. The second is probably the consequence of the first. Previous studies reported similar patterns of physical stratification in our study area, with the upper mix layer on the top of a conspicuous deep salinity maximum right above the top of the thermocline[8, 10, 19]. The upper mix layer of 25 m detected in our study (see Fig.7C) is much shallower than the mean of 62 m reported by[10] for the same region. Salinity certainly caused this shallowness of the mix layer because it increases downwards faster than the decrease of temperature, contributing for the sharp gradients in the pycnocline which facilitates even more the accumulation of chlorophyll rich particles at these layers. In fact the DCML followed closely the 25-26 isopycnals and the associated SSM. It is relevant to mention peak concentrations of chlorophyll between 0,16-0,22 µg.L-1 occurred at salinities ranging from 36 to 36,7 which is slightly above the range of 35- 36 that characterize the nitrate rich SACW[12, 13]. This is consistent with the general assumption that some cianobacteria cells, for instance Prochloroccocus, are unable to use nitrate as source of Nitrogen[5]. Away from islands and seamount slopes the most important source of ammonia for autotrophic cells comes from regeneration in the pelagic microbial food web. Microzooplankton studies made in the same region based on 20 µm net samples[20] and from Niskin bottles specifically at the DCML[21] revealed a highly diversified microzooplankton assemblage made of tintinids, naked ciliates, radiolarians and heterotrophic dinoflagellates. Total densities of these organisms range between 50 and 200 ind. L-1[21] making them important producers of ammonia in tropical oligotrophic waters[5]. The water flow interacting with topography certainly expose bacteria cells to additional ammonia regenerated by benthic organisms. Reference[22] concluded that ammonia regenerated by the benthic community of Caribbean islands accounted for the enhancement of phytoplankton primary production and chlorophyll accumulation in the wake side of the islands. The integrated chlorophyll over 100 meters in the wake of Rocas atoll were in fact higher (12,26 mg.m-2) than upstream of the atoll (8,18 mg.m-2). The same occurred but at a much longer distance downstream of Fernando de Noronha Is. Since bacteria cells use only reduced nitrogen sources recycled in the pelagic system[23, 5], we may argue they will take advantage of additional ammonia recycled by the highly diversified benthic community in the summit of seamounts slopes around Rocas and FN[7]. Considering (i) growth rate of pico-procarionts (e.g., Prochlorococcus) ranges from 0.5 to 1 divisions.day-1[1], (ii) flow speed of the ASEC in our study transect is on average 30-60 cm.s-1[24] and (iii) the ca 27 km distant track over the slope of the atoll with <100 m, we conclude cells just arriving at Rocas will be exposed to additional ammonia for 13 to 20 hours prior to being flushed out to deep waters downstream of the atoll. Nevertheless, with our data we may not conclude if the higher chlorophyll concentrations observed in the wake of Rocas is only due to ammonia fertilization or just accumulation of cells by physical processes. Thickness of the DCML also changed along the horizontal plane. From the easternmost side of the transect (e.g., stn 20) peak chlorophyll concentrations started with 0.21 µg.L-1 decreasing gradually westwards. The DCML at station 17 located over a seamount was not as thick as in the deeper water columns, with maximum concentrations up to 0,1 µg.L-1 near the bottom summit. In contrast to what has been reported over seamounts in the North Pacific[35, 36] uplifting of isopycnals by (e.g.) Taylor columns were not distinguished (see Fig.7C) because it depends on the Coriolis force[38, 39], which is negligible near equatorial latitudes. The 25 isopycnal and the thicker DCML observed downstream as well as upstream of the seamount were limited to depths below its summit. It has been reported significant increase of current velocities and ressuspension of organic particles near the bottom summit of seamounts due to tidal energy and/or internal waves[37, 36]. Except in rare occasions, none of these authors found higher chlorophyll concentrations trapped between specific isopycnals above seamounts. Yet, strong tidal currents over seamounts cause ressuspension of seston and increase dispersion rates, affecting the density of chlorophyll rich cells. To estimate the amount of carbon available from DCML to the benthos of the Rocas atoll, we have to made some assumptions: (i) Chlorophyl:Carbon ratios of 0,0095 to 0,027:1 as proposed by[25] for central Atlantic waters, which is similar to the values found by[26] in the equatorial Pacific, (ii) a 14,8 km wide slope cross section, perpendicular to the currents at the 100 m isobath, (iii) DCML mean thickness of 25 m containing 0,1 to 0,2 µg chlorophyll.L-1 and, (iv) ESW fluxes of 0,13 to 0,21 Sv. Under these conditions, the DCML can provide the atoll ecosystem additional 210 (±170) metric tons of Carbon per day, exclusively from the pelagic primary producers. As stated earlier, the DCML here described follows closely the up and down movement of isopycnals. Internal waves cannot be ignored causing lifting up of pycnoclines over slopes of islands and seamounts. In tropical and sub- tropical oceans, internal waves usually have amplitude >50m, periods from tens of minutes to several hours and wavelengths from hundreds of meters to tens of kilometers; the orbital motion of water and particles have the largest diameter at the pycnocline and decreases up and downwards [32]. Internal waves have been detected year-round in the Western Equatorial Atlantic off northeast Brazil but more frequently between December and March by[27, 28]. The first authors detected internal waves every 11.4 to 13.8 hours between 30-78 meters, the second reported waves ranging usually from 5-8 meters high but 9 waves 20-23 m high were measured during a 18 days survey from March to April, i.e., during the same seasonal period of our investigation. Depths of pycnocline ranged for approximately 45 meters along our sampling transect (Fig.6). The top layers of the nutrient-rich SACW on the wave's crest may reach up to 75 m, which according to[14] correspond more or less to the 1% of surface light in this area. It has been demonstrated a strong diel variation in the expression of photosynthesis genes of Prochloroccocus collected in different diurnal periods in the equatorial Atlantic[29]. In a fixed position, internal waves may expose autotroph bacteria to highly variable light intensities. Reference[30] made similar assumptions regarding light intensification due to the lift of the pycnocline and the associated chloro- phyll maximum at Cobb Seamount in the Northeast Pacific. Since equatorial tidal currents are stronger and the potential for internal waves action against the slopes is great one may expect similar processes may account for the horizontal differences of chlorophyll concentrations near seamounts and slopes along the FNR. The annual rate of production of pico-photoautotrophs in oligotrophic oceans is constant and able to balance losses by grazing[31]. This is certainly what keeps the chlorophyll maximum throughout the year. Yet the peak chlorophyll concentrations here detected differed by two to three folds. Reference[1] reported a two-fold difference in depthintegrated cell densities of Prochloroccocus between afternoon and midnight in the equatorial and subtropical Pacific. Since our stations were sampled during the day and night, differences of peak chlorophyll concentrations along the DCML might have been associated with sampling time. Environmental controls derived from the interaction of water flow with topography along the FNR is complex and affect the balance between cell growth and losses that maintain DCML. Uplifting of isopycnals by internal waves, changing light regime, and additional sources of nutrients, particularly ammonium from benthic communities, play a significant role on the dynamics of DCML and deserve a more investigation on time-spatial scales not covered here.
ACKNOWLEDGEMENTS
- To Dr. S. Gruber, chief scientist of the expedition, for the invitation to this cruises. Thanks are also due to the crew of the R/V Seward Johnson, for its support during field work. This work was supported by the Department of Biological Sciences of the State University of Santa Cruz (Bahia) and the Department of Systematics and Ecology of the Federal University of Paraíba.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML