-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Marine Science
p-ISSN: 2163-2421 e-ISSN: 2163-243X
2012; 2(6): 125-131
doi: 10.5923/j.ms.20120206.03
The Effect of Nutrient Enrichment on three Species of Macroalgae as Determined by Photoacoustics
Yulia Pinchasov-Grinblat 1, Razy Hoffman 1, Stefano Goffredo 2, Giuseppe Falini 3, Zvy Dubinsky 1
1The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan 52900, Israel
2Marine Science Group, Department of Evolutionary and Experimental Biology, Alma Mater Studiorum, University of Bologna, Via F. Selmi 3, I-40126, Bologna, Italy, European Union
3Department of Chemistry ‘G. Ciamician’, Alma Mater Studiorum-University of Bologna, Via F. Selmi 2, I-40126, Bologna, Italy, European Union
Correspondence to: Yulia Pinchasov-Grinblat , The Mina & Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan 52900, Israel.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The efficiency of photorophs in storing light energy in products of photosynthesis, or the quantum yield of the process, determines the flux of energy into any ecosystem, and is readily affected by any stress factor, physiological or environmental. Hence it can serve as a sensitive reporter of imminent ecosystem perturbation. Current methods of estimating that parameter are either indirect, or tedious and labor consuming. The photoacoustic method is based on the direct sensing of the variable fraction of light energy degraded to heat, that while absorbed by the plant cells or tissue, failed to be stored as energy rich photosynthate. Algae are the main primary producers in all water bodies, marine, freshwater, natural and manmade, and as such they provide the energy basis for all aquatic ecosystems. The biomass and vitality of the algal population responds rapidly to seasonal changes in environmental factors such as temperature, light, vertical mixing, eutrophication, pollution and nutrient limitation. Typical processes resulting from human activities such as non-point sources of agrochemicals, sewage outflows, and effluents from food, animal and other chemical processing industries as well as from urban and rural runoff, contribute considerable amounts of nitrogen and phosphorous, in many cases in addition to heavy metals, to natural and artificial water bodies. In this study, we examined by the photoacoustic method the effects of enrichment by nitrogen and phosphorus on the photosynthetic efficiency of three common Mediterranean seaweed species which were collected from the field. The application developed by us, allows the rapid and direct determination of phytoplankton photosynthetic activity, by relating the energy stored photochemically to the total light energy absorbed by the plant material. The aim of this work was the examination of the applicability of the photoacoustic system to ecological work with macroalgae, since until now it has been successfully used only in homogenous phytoplankton cultures.
Keywords: Photoacoustics, Photosynthesis, Macroalgae, Nutrient Enrichment
Cite this paper: Yulia Pinchasov-Grinblat , Razy Hoffman , Stefano Goffredo , Giuseppe Falini , Zvy Dubinsky , "The Effect of Nutrient Enrichment on three Species of Macroalgae as Determined by Photoacoustics", Marine Science, Vol. 2 No. 6, 2012, pp. 125-131. doi: 10.5923/j.ms.20120206.03.
Article Outline
1. Introduction
- The seasonal changes in algae populations are brought about by the annual rhythms of temperature, light intensity, and enrichment or depletion of nutrients in surface waters[1].Under sufficient light intensity, photosynthesis of phytoplankton is limited primarily by the availability of essential nutrients, most commonly nitrogen and phosphorus[2-4]. Therefore, any increase or decrease in the supply of these nutrients results in dramatic changes in the concentration of phytoplankton[1],[5]. Such changes in nutrient concentrations, may stem from natural events like mixing of water bodies and upwelling currents, or from anthropogenic sources like marine and lacustrine pollution[5].Since even a barely detectable increase in nutrients is amplified by the phytoplankton into an easily quantifiable change in their total biomass and taxonomic composition, phytoplankton are sensitive pollution detectors. The capability to harvest nutrients from the external environment at both high and low concentrations is an important property of the ecology of algae. These changes in biomass and productivity have been traditionally recorded by measurements of absorption, fluorescence, oxygen evolution, and 14C labeling[6],[7]. The potential of photoacoustics in quantifying and characterizing algae photosynhtesis and chlorophyll concentration had been reported [6],[7]. The photoacoustic method is based on the conversion of the light energy into heat. The application developed by us, allows the rapid and direct determination of algae photosynthetic activity, by relating the energy stored photochemically to the total light energy absorbed by the plant material. For detailed description see “Methods and Materials”. Seaweeds are “bentical algae” that live in the sea . [8]. Seaweeds are macroalgae which are distributed in three phyla: Chlorophyta which are often called green algae (~1200 species), Rhodophyta, the red algae (~6000 species) and Heterokontophyta or brown algae (~1750 species)[9].Seaweeds grow in the photic zone which is in the range between the surface water levels (in the intertidal zone) and down to ~200 meters deep in the open ocean[10]. Ulva rigida C. Agardh, Hypnea musciformis (Wulfen in Jacquin) J.V. Lamouroux and Padina pavonica (Linnaeus) Thivy Agardh are green, red and brown algae respectively. These three macroalgae are very common along the Israeli shore and mainly grow on the Kurkar intertidal abrasion platforms. The rationale for the species selection was to compare the effects of the unique laser used previously[11],[12] to estimate photosynthesis efficiency only on phytoplankton , on species of macroalgae from three different phyla that have different types of thallus morphologies and diverse pigmet assortments. Ulva rigida has green flat sheet-like thallus with toothed margins. This thallus is composed of two cell layers[13]. Hypnea musciformis has red-green narrow, cylindrical, branched thallus morphology. Its branch apices are slightly upcurved, flattened hooks[14]. Padina pavonica is characterized by flat calcified "ear-like" blades which has circinnately inrolled apical margins[15].The aim of this work was the examination of the applicability of the photoacoustic system, previously used only in homogenous phytoplankton cultures, to ecological work with macroalgae.
2. Methods and Materials
2.1. Photoacoustics
- The photoacoustic method is based on the conversion of absorbed light to heat. When the energy of the photons absorbed in a sample is degraded to heat the thermal expansion of the material causes a volume increase:
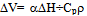 | (1) |
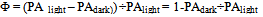 | (2) |
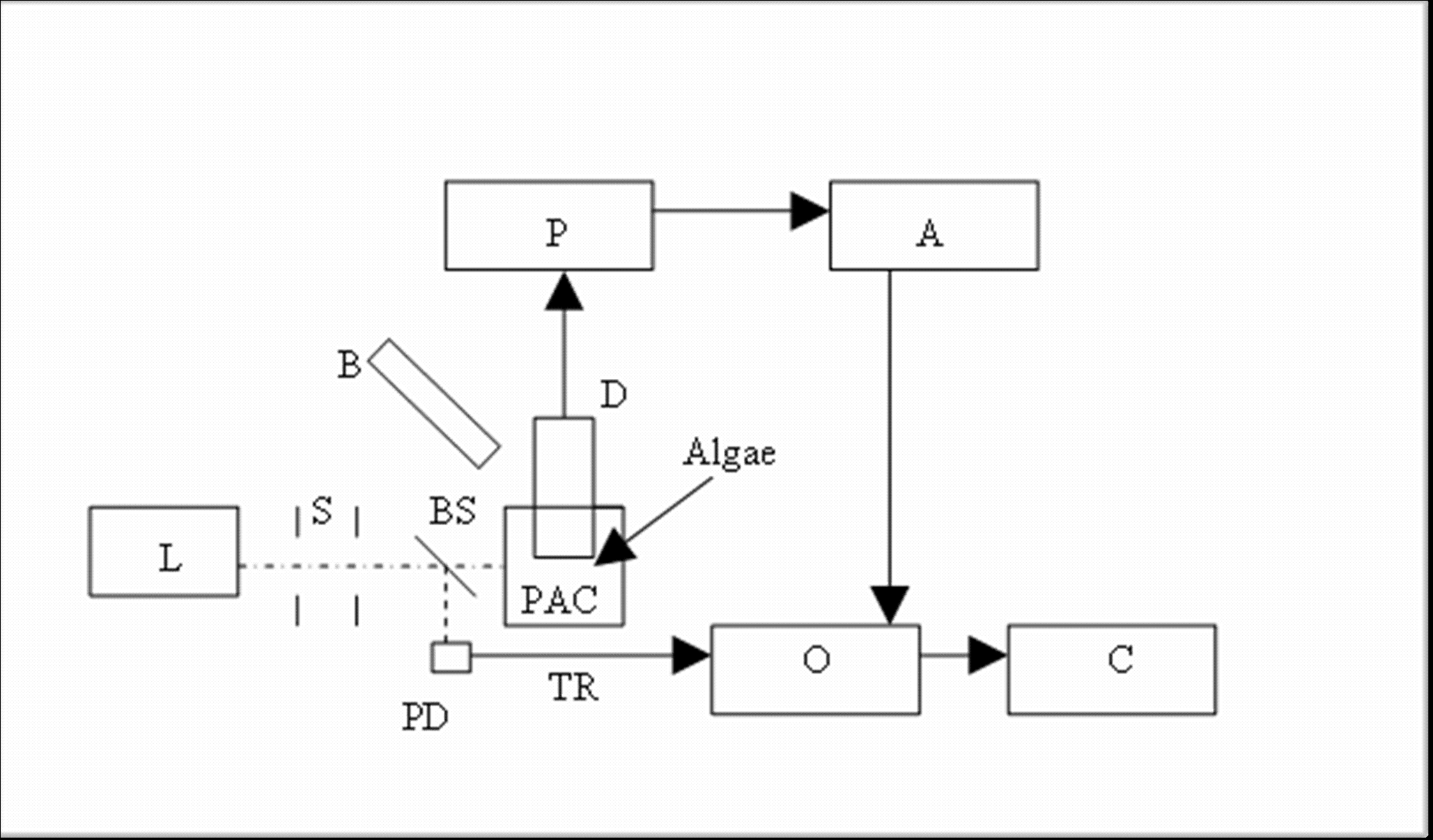
- The experimental setup is shown schematically in Figure 1. The overall procedure was similar to that described in detail in Pinchasov et al.[11]. The second harmonic of a Continuum Minilite Q-Switched Nd-Yag laser at 532 nm was used. The signal was processed with a Tektronix TDS 430A oscilloscope. The submersible, stainless steel enclosed homemade detector contained a 10 mm diameter resonating ceramic disc (BM 500, Sensor, Ontario, Canada). The sample was placed in a 16 mm quartz glass cell (PAC). The laser (L) pulse, after passing through a pair of 1 mm wide slits (S) is incident upon the suspension of algae whose pigments absorb part of the laser light. Depending on the experimental conditions a variable fraction of the absorbed light pulse is stored in the products of photosynthesis. The remainder of the absorbed light is converted to heat producing an acoustic wave. This is intercepted by a detector (D), containing the above ceramic disc. A small portion of the laser pulse is deflected by a beam splitter (BS) and used to trigger the Tektronix TDS 430A oscilloscope, where the amplified (Amptek A-250 Preamp and Stanford Research A 560 Amp) photoacoustic signal is recorded. The signal contains a noisy background and later reflections from the walls of the vessel as well as from impedance mismatch within the detector. Signal to noise was improved by averaging over 128 pulses, and by taking RMS values over the time of the recorded signal (~10 µs). Weak (~20 µJ cm-2), 5ns pulses at 532 nm wavelength, were used as a probe for ongoing photosynthesis. The source of the background light (B), was a quartz-halogen illuminator (Cole-Parmer 4971). The intensity of the background light was adjusted to the 2000 E m-2 s-1 by neutral density filters and measured with a LiCor light meter equipped with a cosine quantum sensor (for technical details see Pinchasov et al.[11]).
2.2. Algae Samples
- The samples of three common macroalgae species: Ulva rigida, Hypnea musciformis and Padina pavonica were collected from the intertidal abrasion platforms at Bat Yam (located in the middle of the Israeli Mediterranean) during spring 2010. All samples were kept at 22 ± 0.1℃ in 100 mL Erlynmayers during 192 hours under continuous irradiance at ~200 ± 5.0 µI m-2 s-1. Initial volume of medium in Erlynmayers was 75 mL. For every species were processed 3 duplicate samples.Chlorophyll was determined by photoacoustic method, based on the proportionality of the photoacoustic signal to the amount of pigment. For all experiments (include control) were used slices of common. The samples were exposed to 3 treatments: nitrogen (was added as NaNO3, 10 mL at concentration of 50 ml/L from the stock of 75.0 g/L), phosphorus (was added as NaH2PO4,10 mL at concentration of 5 ml from the stock of 5.0 g/L), and nitrogen and phosphorus together. Controls were kept in seawater alone.
3. Results and Discussion
- We have used the effects of nutrient limitation and of eutrophication on the photosynthetic efficiency of seaweeds as experimental variables in a demonstration of the applicability of photoacoustics in aquatic ecology. Nutrient limitation on the one hand and anthropogenic eutrophication on the other, are among the most important factors determining the overall ecological status of water bodies. Since nutrient status is known to rapidly affect algal growth and photosynthesis we chose that environmental factor for our studyIn general, in all samples (Figs. 2, 3 and 4), photosynthetic efficiency and chlorophyll concentration (photoacoustic signal) decreased with time, during the 192 hours of incubation.. As is evident from Figures 2, 3 and 4, the photoacoustic signal rapidly decreased , and within 190 hours, the controls declined to some 70 % in P. pavonica (Fig. 2) and H. musciformis (Fig. 4), and ~ 50 % in U. rigida (Fig. 3), of the initial values. The addition of nutrients slowed down, but did not prevent, such decline (~ 20 % in U. rigida (Fig. 3) and ~ 50 % and 30 % in P. pavonica and H. musciformis, respectively (Figs. 2 and 4). The decline in all treatments, including in the P and N enriched ones is likely due to the requirement for additional nutrients besides nitrogen and phosphorus.Similar effects of nutrient limitation on photosynthetic apparatus and chlorophyll concentration have been reported by Villares et al.[20] and Litchman et al.[21]. Our results suggest that the added nitrogen prevented this nutrient from becoming limiting, and, therefore, the observed declines in photosynthesis and chlorophyll were due to the incipient phosphorus limitation.Samples with added nitrogen and phosphorus together were usually the slowest to show a decrease in photosynthetic efficiency. A decrease in chlorophyll concentration under nutrient limitation has been observed for both marine and freshwater algae[21-23].Reduction trend in chlorophyll concentration in nutrient-enriched samples may be explained by the availability of nutrients required for the synthesis of pigments (mostly nitrogen), and membranes (both nitrogen and phosphorus).The general conclusion from these data, which was obtained by photoacoustics, is that in the sampled algae, nitrogen was the first nutrient that became limiting followed by phosphorus. In all cases the addition of phosphorus and nitrogen alone, or together slowed down the decline in photosynthetic efficiency, that was the fastest in the unsupplemented controls.Our results, which were obtained by the photoacoustic method, agree with others, previously described in literature [24-26] showing that the nitrogen and the phosphorus additions mitigated the effects of nutrient limitation.Our results show that photoacoustics can be a useful tool in ecophysiological studies of macroalgae, providing sensitive and immediate evidence for any deleterious processes affecting seaweeds. Based on our results we are working on expanding the method's applicability to various additional phototrophs such as seagrasses, turf algae, algal mats and zooxanthellate corals..The directness of our method holds the potential for development of novel tools for monitoring changes in photosynthetic efficiency related to water quality in marine and freshwater benthos communities, The photocoustic method can provide early warning alerts to incipient negative ecosystem developments, as well as report recovery processes in response to the implementation of remediation measures
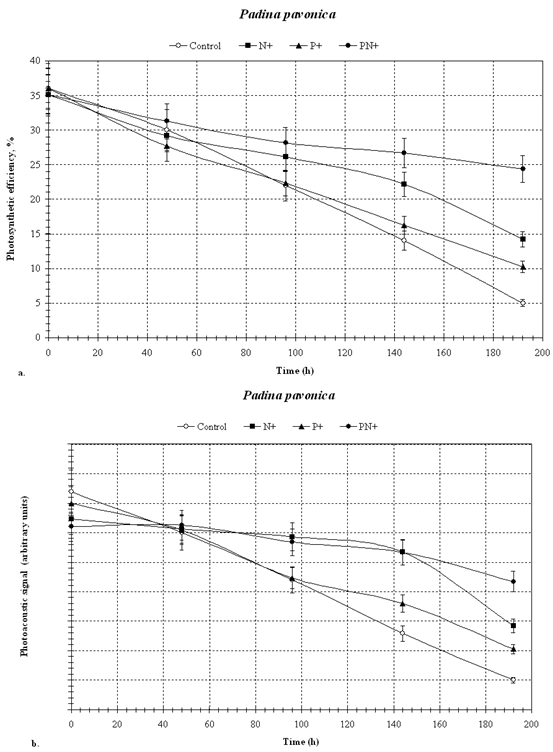 | Figure 2. P. pavonica – photosynthesis (a) and chlorophyll (b) by photoacoustics |
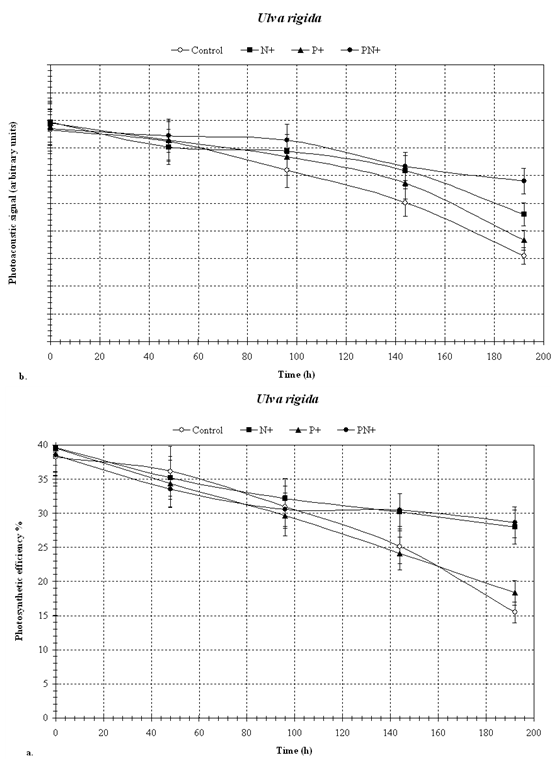 | Figure 3. U. Rigida – photosynthesis (a) and chlorophyll (b) by photoacoustics |
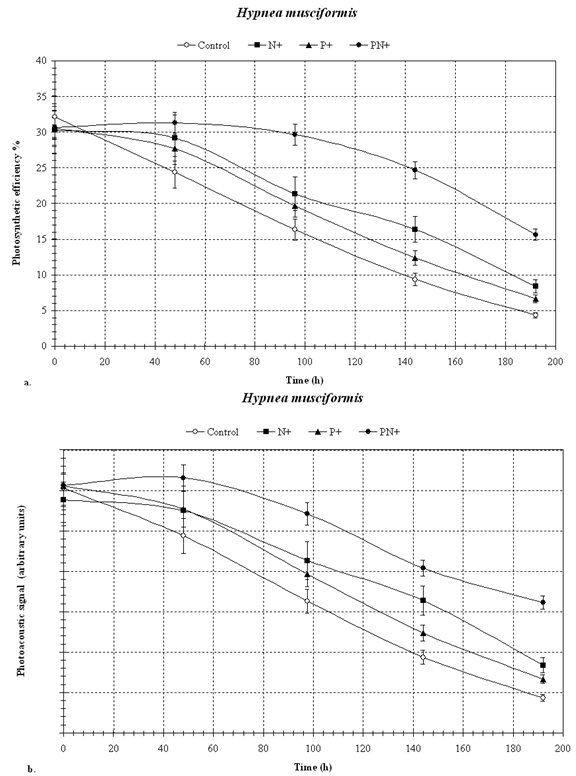 | Figure 4. H. musciformis – photosynthesis (a) and chlorophyll (b) by photoacoustics |
ACKNOWLEDGMENTS
- This research was supported by grant European Research Council 2009-AdG - 249930 to Z. D. and G. PThis research was supported by grant EU FP7 Europen Reseacrh Council 2012 – 309646 to Z. D.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML