-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Marine Science
p-ISSN: 2163-2421 e-ISSN: 2163-243X
2012; 2(6): 120-124
doi: 10.5923/j.ms.20120206.02
Zooplankton Abundance and Composition in Surf Zone of Gopalpur Port, East Coast of India-A Case Study
Biraja Ku. Sahu , S. K. Baliarsingh , S. Srichandan , K. C. Sahu
P. G. Dept. of Marine Sciences, Berhampur University, Ganjam, 760007, Odisha,
Correspondence to: K. C. Sahu , P. G. Dept. of Marine Sciences, Berhampur University, Ganjam, 760007, Odisha,.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Zooplankton abundance, biomass and composition were studied by quantitative approach in coastal water of Gopalpur Port in two selected stations. From the study we recorded a total of 28 zooplankton groups/taxa of which the copepods formed the dominant group in both the stations. The other dominant groups were chaetognaths, decapod larvae, gastropods, bivalve larvae, cladocera, amphipod etc. In the study area, the maximum biomass at 11.9 ml/100m3 and the minimum at 8.6 ml/100m3 were recorded. The maximum and minimum densities of zooplankton were at 17389 Nos./100m3 and 13425 Nos./100m3 respectively. There was not any significant difference in zooplankton abundance between stations (p> 0.05) and within sampling months (p>0.05).
Keywords: Zooplankton, Biomass, Abundance, Gopalpur Port
Cite this paper: Biraja Ku. Sahu , S. K. Baliarsingh , S. Srichandan , K. C. Sahu , "Zooplankton Abundance and Composition in Surf Zone of Gopalpur Port, East Coast of India-A Case Study", Marine Science, Vol. 2 No. 6, 2012, pp. 120-124. doi: 10.5923/j.ms.20120206.02.
Article Outline
1. Introduction
- Zooplankton plays a major role in functioning and productivity of aquatic ecosystems through its impact on the nutrient dynamics and its unique position in the food web. In reverse, they can also act as an important disease reservoir. Many species of zooplankton can be used as biological indicators for water pollution, water quality and eutrophication[1-4]. Zooplankton communities are highly influenced by spatio-temporal variations in hydrochemical parameters and physical forces[5-7]. Species composition and seasonal variation in plankton densities are important in determining the trophic level of an ecosystem. Information dealing with the plankton of the coastal waters Orissa, that is of zooplankton are meager and mostly limited to Chilika lake[8-10], Rushikulya estuary[11], Bahuda estuary[12-13], Burhabalanga estuary[14] and coastal waters of Bay of Bengal. As the study area is under the influence of anthropogenic pressure by means of receiving pollutants from Gopalpur Port and nearby industries, the output of this study will act as a reference for coastal researchers and environmental planners for environmental impact assessment purpose.
2. Materials and Methods
- The present study region, coastal water of Gopalpur Port (Figure 1) is in the south of Odisha coast between latitudes 19.300° N - 19.308° N and longitudes 84.962° E - 84.972° E. Here beach material is purely sandy. Rainfall in this area though concentrated in monsoon seasons with peaks in July, rain due to tropical cyclones, storm surges and deep depressions are common. Activities of Gopalpur Port for all weather capability, regular heavy mineral exploration by Indian Rare Earths Limited, agricultural runoff, oil spills due to regular mechanized fishing boat movements and future anthropogenic influence draw attention to document the inventory and status of zooplankton of this dynamic area.
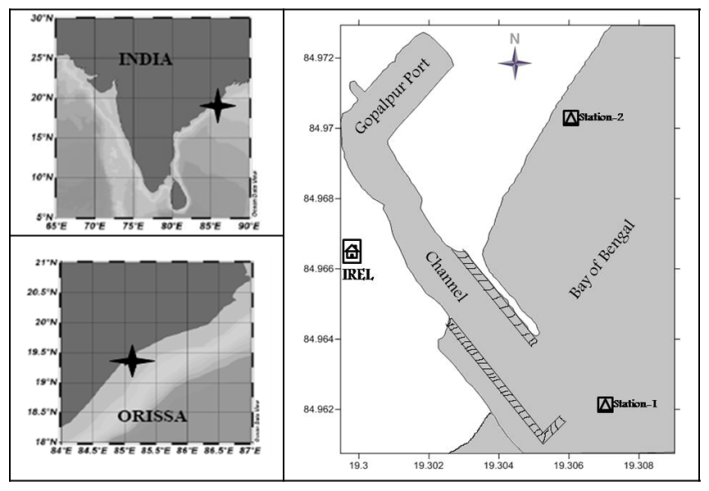 | Figure 1. Map showing sampling locations in numerical figures (1 and 2) |
3. Results and Discussion
- The term biomass symbolizes the live weight of living matter present in the zooplankton sample. The value obtained is used to evaluate the secondary productivity and fishery potential of the study area. Amongst three months of data, the maximum biomass was obtained in December with magnitude of 11.9 ml/100 m3 (Figure 2). The minimum was obtained in the month of October with 8.6 ml/100 m3. Highest biomass of 12.2 ml/100m3 was found in Station 1 during December. It was found that higher biomass was found in Station 1 as compared to Station 2 (Figure 2). In three months study, the maximum average density of zooplankton was obtained in December with the value 17389 Nos./100 m3 and lowest density in October with the value 13425 Nos./100 m3. Highest zooplankton density of 18712 Nos./100 m3 was found in Station 2 in December (Table-1). A total number of 28 diverse groups / taxa were identified from the zooplankton samples of the study area. The groups contain foraminifera, siphonophore, medusae, ctenophore, chaetognatha, polychate, copepoda, cladocera, ostracoda, amphipoda, isopoda, cyprid larvae, decapod larva (zoea and megalopa larvae), Lucifer spp, mysids, prawn larvae, stomatopoda, brachiopoda larvae, gastropods, bivalve larvae, heteropoda, pteropoda, ophiopluteus larvae, doliolum, salp, appendicularia, fish larva, fish egg etc (Table-1). The highest number of groups i.e. 22 was found in November followed by 21 in December. Normally, higher number of groups was found in Station 1 as compared to Station 2.
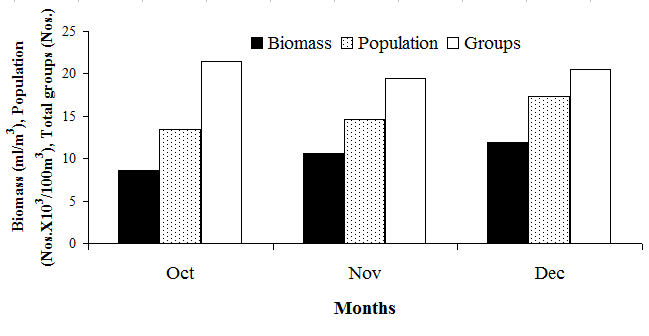 | Figure 2. Zooplankton biomass, population density and groups in the study area |
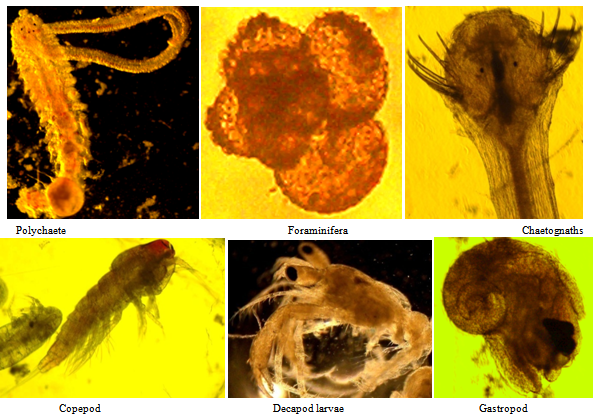 | Figure 3. Under microscope photographs of some important dominant zooplankton groups |
|
4. Conclusions
- In the present piece of study, 28 zooplankton groups/taxa have been documented of which the copepods formed the dominant group in both the stations. Zooplankton play an important role in forming a main link between primary producers (phytoplankton) and higher trophic levels[27-28]. During the study period, it is further established that there was no significant difference in zooplankton composition in between stations and within the sampling months. Previously it has been reported that zooplankton diversity weakens due to domestic sewages and industrial wastes[29]. In recent days, the ongoing industrial activities by TATA steel and construction of Gopalpur all weather port since are gaining their momentum, zooplankton fauna will appear to be more prone towards anthropogenic threats. It is a fact that zooplankton diversity in an area regulates the fishery, an important sector of the livelihood for many coastal inhabitants. Their community composition therefore should be studied on regular basis and appropriate measures should be taken for eco-restoration in case of any environmental threat caused to these tiny denizens. The present study revealed the faunal composition of zooplankter only for post-monsoon months. But environmental drivers responsible for the season-wise fluctuation in zooplankton abundance and species composition should further be studied in relation to environmental and anthropogenic factors.
ACKNOWLEDGEMENTS
- The present work was carried out as a part of INCOIS (Indian National Centre for Ocean Information Services) funded SATCORE (Satellite Coastal and Oceanographic Research) project awarded to Berhampur University. All the authors are highly indebted to Dr. T.S Kumar and Dr. A. A Lotliker of INCOIS for their suggestions during the course of study.
References
| [1] | D. Bonnet, C. S. Frid, “Copepod species considered as indicators of water mass influence and changes: results from a Northumberland coastal station”, ICES. J. Mar. Sc., vol. 61, pp. 485-491, 2004. |
| [2] | C. H. Hsieh, T. S. Chiu, C. T. Shih, “Copepod diversity and compositions as indicators of intrusions of the Kuroshio Branch Current into the northern Taiwan Strait in spring 2000”, Zool. Stud., vol. 43, no. 2, pp. 393-403, 2004. |
| [3] | J. S. Hwang, S. Souissi, H. U. Dahms, L. C. Tseng, F. G. Schmitt, Q. C. Chen, “Rank-abundance allocations as a tool to analyze planktonic copepod assemblages off the Danshuei Estuary (Taiwan)”, Zool. Stud., vol. 48, no.1, pp. 49-62, 2008. |
| [4] | L. C. Tseng, R. Kumar, H.U. Dahms, Q. C. Chen, J. S. Hwang, “Monsoon driven seasonal succession of copepod assemblages in the coastal waters of the northeastern Taiwan Strait”, Zool. Stud., vol. 47, no. 1, pp. 46-60, 2008. |
| [5] | F. Bianchi, F. Acri, A. Bernardi, A. Berton, A. Boldrin, E. Camatti, D. Cassin, A. Comaschi, “Can plankton communities be considered as bioindicators of water quality in the lagoon of Venice?”, Mar. Pollut. Bull., vol. 46, pp. 964-971, 2003. |
| [6] | S. H. Hsiao, C. Y. Lee., C. T. Shih, J .S. Hwang, “Calanoid copepods of the Kuroshio Current east of Taiwan, with a note on the presence of Calanus jashnovi Hulseman (1994)”, Zool. Stud., vol. 43, no. 2, pp. 323-331, 2004. |
| [7] | R. T. T. Sridhar, S. S. Kumar, L. Kannan, “Water quality and phytoplankton characteristics in the Palk Bay, southeast coast of India”, Journal of Environ. Biol., vol. 27, no. 3, pp. 561-566, 2006. |
| [8] | S. Patnaik, “Observations on the seasonal fluctuations of plankton in the Chilka Lake”, Indian J. Fish., vol. 20, pp. 43-55, 1973. |
| [9] | R. B. S. Sewell, “Notes on plankton from the Chilka Lake”, Rec. Indian Mus., vol. 9, pp. 338-340, 1913. |
| [10] | M. P. Devasundaram, J. C. Roy, “A preliminary study of the plankton of the Chilka Lake for the years 1950 and 1951”, in IPFC Publication symposium on marine and fresh water plankton in the Indo-Pacific, 1954, p. 1-7. |
| [11] | R. Gouda, R. C. Panigrahy, “Zooplankton distribution in Rushikulya estuary”, Journal of Aquacult. Tropics, vol. 10, pp. 201-211, 1995. |
| [12] | S. Mishra, R. C. Panigrahy, “Copepods of Bahuda estuary (Orissa), east coast of India”, Indian J. Mar. Sci., vol. 25, pp. 98-102, 1996. |
| [13] | S. Mishra, R. C. Panigrahy, “Zooplankton ecology of the Bahuda estuary (Orissa), east coast of India”, Indian J. Mar. Sci., vol. 28, pp. 297-301, 1999. |
| [14] | N. Ramaiah, A. Chatterji, M. Madhupratap, “A study on the zooplankton of the Burhabalanga estuary, Orissa coast”, in Proceedings of Indian National Science Academy, vol. 62, pp.1-4, 1996. |
| [15] | L. R. Kasturirangan, “A key to the identification of the more common planktonic Copepoda of Indian coastal waters”, Indian National Committee on Oceanic Research, Council of Scientific and Industrial Research, New Delhi, no. 2, 1963, 87p. |
| [16] | G. E. Newell, R. C. Newell, “Marine Plankton - A Practical Guide”, Hutchinson Educational Ltd., London, 1977, 244 p. |
| [17] | D.V.P. Conway, R. G. White, J. Hugues-Dit-Ciles, C. P. Gallienne, D. B. Robins, “Guide to the coastal and surface zooplankton of the south-western Indian Ocean, Occasional Publications”, Marine Biological Association of the United Kingdom, vol. 15, 2003, 354p. |
| [18] | G. Sahu, A. K. Mohanty, B. Singhasamanta, D. Mahapatra, R. C. Panigrahy, K. K. Satpathy, B. K. Sahu, “Zooplankton Diversity in the Nearshore waters of Bay of Bengal, Off Rushikulya Estuary”, IUP Journal of Env. Sci., vol. 4, no. 2, pp. 61-85, 2010. |
| [19] | U. Krumme, T. H. Liang, “Tidal-Induced Changes in a Copepod-Dominated Zooplankton Community in a Macrotidal Mangrove Channel in Northern Brazil”, Zool. Stud., vol. 43, no. 2, pp. 404-414, 2004. |
| [20] | B. Beyst, D. Buysse, A. Dewicke, J. Mees, “Surf zone hyperbenthos of Belgian sandy beaches: Seasonal patterns”, Estuarine, Coastal Shelf Sci., vol. 53, pp. 877-895, 2001. |
| [21] | A. Sampey, A. D. McKinnon, M. G. Meekan, M. I. Mc Cormick, “Glimpse into guts: overview of the feeding of larvae of tropical shore”, Mar. Ecol. Prog. Ser., vol. 339, pp. 243-257, 2007. |
| [22] | G. Padmavati, C. Goswami, “Zooplankton ecology in the Mandovi-Zuari estuarine system of Goa, west coast of India”, Indian J. Mar. Sci., vol. 25, pp. 268-273. 1996. |
| [23] | R. Koppelmann, H.Weikert, “Transfer of organic matter in the deep Arabian Sea zooplankton community: insights from δ15N analysis”, Deep-Sea Res. vol. II, no. 47, pp. 2653-2672, 2000. |
| [24] | S. N. Gajbhiye, J. Ram, V. R. Nair, B. N. Desai, “Plankton of the Narmada estuary and adjacent creeks”, Mahasagar, vol. 14, pp. 23-31, 1981 |
| [25] | M. D. Zingde, N. B. Bhosale, P. V. Narvekar, B. N. Desai, “Hydrography and water quality of Bombay Harbour”, in Prakash, R. (ed) Environment Strategies and Biosciences, Society of Biosciences, Muzafarnagar, India, pp. 37-58, 1989. |
| [26] | J. Urban-Rich, M. Dagg, J. Peterson, “Copepod grazing on phytoplankton in the Pacific sector of the Antarctic Polar Front”, Deep Sea Research Part II: Topical Studies in Oceanography, vol. 48, no. 19, pp. 4223–4246, 2001. |
| [27] | J. E. G. Raymont, “Plankton and productivity of the Oceans”, Pergamon Press, vol. 2, 1980. |
| [28] | S. F. Timofeev, “Ecology of the marine zooplankton (in Russian)”, Murmansk State Pedagogical Institute Press, Murmansk, 2000. |
| [29] | M. Madhupradap, P. Haridas, “Epipelagic calanoid copepods of the northern India”, Oceanol. Acta, vol. 9, pp. 105-107, 1986. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML