-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Marine Science
p-ISSN: 2163-2421 e-ISSN: 2163-243X
2012; 2(2): 7-12
doi: 10.5923/j.ms.20120202.02
Characterization of PhotoprotectiveCompounds in Marine Zooplankton of the Southwest Coast of India: An Ecological Perspective
Nallathambi T 1, Robin R. S 2, K.Vishnu Vardhan 2, Pradipta R. Muduli 2
1Vessel Management Cell, National Institute of Ocean Technology, Chennai, 600100, India
2ICMAM Project Directorate, Ministry of Earth Sciences, NIOT Campus, Pallikaranai, Chennai, 600100, India
Correspondence to: Nallathambi T , Vessel Management Cell, National Institute of Ocean Technology, Chennai, 600100, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
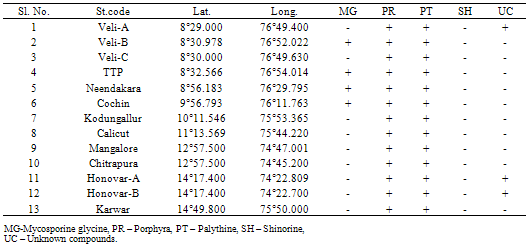
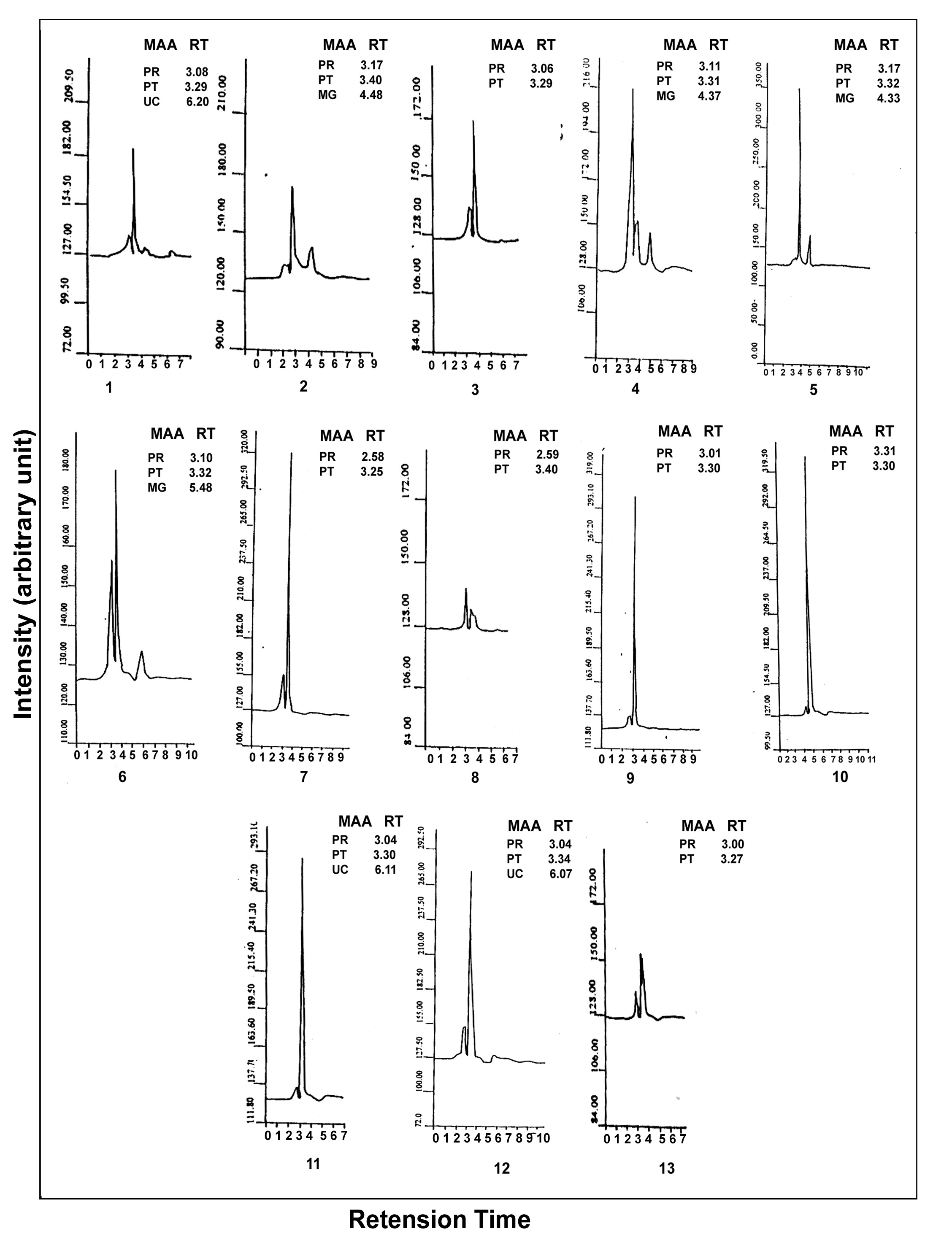
Inthe marine environment, organisms are under threat due to several environmental fluctuations such as ultraviolet (UV) radiation, coastal pollution etc. However, it is encouraging to note that the marine organisms try to protect themselves from UV-radiation by synthesizing photprotective compounds. Since phytoplankton can synthesize the UV-protecective compounds and are grazed by zooplankton, we investigated the presence of photoprotective compounds in zooplankton. In this context, a study has been conducted for the first time in the shelf waters of the southwest coast of Arabian Sea, India for the characterization of photoprotective compounds in the zooplankton community. The study revealed the presence of photoprotective compounds, such asporphyra and palythine in the zooplankton community of all the stations, in addition to mycosporine glycine, in the polluted waters of the stations,Veli, Neendakari and Cochin.
Keywords: Arabian Sea, Mycosporine like amino acids, zooplankton, UV-protective compounds, antioxidant metabolites
Article Outline
1. Introduction
- Impact of UV- radiation on aquatic food webs has been stimulated by realizing the increasing levels of UV- radiation reaching earth’s surface[1]. Despite the low intensity at the ground level, it causes biological damage because of the high-energy content of photon, and the DNA damage via the formation of cyclobutane and pyrimidine dimers[2-6]. One possible strategy by the organisms to protect themselves against UV-radiation is by the synthesis of UV-absorbing compounds that act as natural sunscreens. One such compound, mycosporine, identified in fungi was found to have a role in UV-induced sporulation[7-10]. Mycosporine like amino acids (MAAs) are found in marine organisms, from bacteria to fish[11, 12] and in terrestrial microorganisms like fungi[13]. High concentrations of MAAs are found in epilithiccyanobacterial or algal mats[14, 15]. Accumulation of MAAs has also been reported for the population of the copepod, Boeckellatiticacae from the tropical high altitude Lake Titicaca[16]. Concentration of MAAs in the population of Cyclops abyssorum and C.abyssorumtatricus from the lakes in the Alps has been found to increase exponentially with lake elevation and underwater UV transparency. Copepods of alpine lakes also accumulate high amounts of carotenoids that give them the intense red appearance and provide with added protection[17-19]. Concentration of these compounds in the calanoid copepods was found to be increased with lake elevation and shallowness of the system, thus establishing a direct correlation between the concentration and the UV-intensity[20]. Suchenvironmental factors besides UV exposure are also important in regulating the synthesis of MAAs[21].During the past two decades, a substantial loss in the stratospheric ozone layer has been noticed that has aroused interest in studying the effects of increased ultraviolet radiation (UVR), particularly UV-B radiation (280–315 nm), on the earth’s surface. Solar UV-B radiation is detrimental to most sun-exposed organisms, including humans[22]. An increase in UV-B radiation has led to search for the natural photoprotective compounds from various organisms such as microorganisms, plants and animals of marine as well as freshwater ecosystems. A number of photoprotective compounds, such as melanins, MAAs, scytonemin, parietin, usnic acid, carotenoids, phycobiliproteins, phenylpropanoids and flavonoids and several other UV-absorbing substances of unknown chemical structure have been identified from different organisms[23-25].There have been a number of reviews about diverse classes of compounds from natural sources, including marine habitats, but the occurrence of photoprotectants from marine sources has only partially been elucidated. Ultraviolet radiation (UVR) is one of the most harmful exogenous agents and may affect a number of biological functions in all sun exposed living organisms. Solar radiation exposes the organisms to harmful doses of UV-B and UV-A (315–400 nm) radiation in their natural habitats. In response to intense solar radiation, organisms have evolved positive mechanisms such as avoidance, repair and protection by synthesizing or accumulating a series of photoprotective compounds, such as MAAs, scytonemin, carotenoids and certain other compounds to counteract the toxicity of UV (particularly UV-B) radiation[26-29]. Furthermore, MAAs is the most common compounds with a potential role as UV sunscreens in marine organisms. It has been found that MAAs provides protection from UVR not only for their producers, but also to primary and secondary consumers through the food chain[30].Production of MAAs from marine zooplankton requires holistic understanding the transfer of MAAs from primary to higher trophic levels through the marine food chain. Findings during this investigation are considered important since much of our knowledge on MAAs is from the fresh water habitat and temperate waters. Studies on the MAAs production from the shelf waters of tropical environments are lacking. Hence, an attempt was made for the first time in this regard along the shelf waters of southwest coast, India. This study investigated the relationship among zooplankton, chlorophylla, productivity and photoprotective compounds characterization in zooplankton community with the existing environmental conditions.
2. Materials and methods
- Study was carried out at thirteen stations along (~ 1400 km stretch between 8°N and 14°N lat) the shelf waters of the southwest coast of Arabian Sea, India, in which eight stations were selected from off Kerala coast and five from off
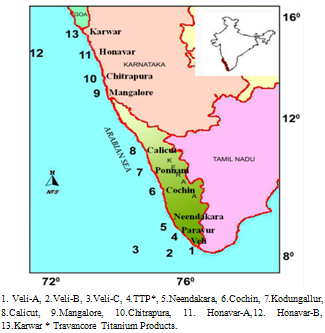 | Figure 1. Study area map with sampling locations |
3. Results and Discussion
- Variations in hydrographic characteristics along southwest waters were presented in (Figure 2 a-d).South west coast has a warm humid climate with ~32oC air temperature. Water column remained relatively cool (avg. 28.16 ± 0.73°C) except station Veli. Salinity is one of the prime factors, which influences the abundance and distribution of the fauna and flora in the coastal waters. Salinity (avg. 28.94 ± 5.59) showed a wide fluctuation at stations proximal to estuaries. Low pH recorded at southern stations (Veli, Paravur, Neendakara) showed a great fluctuation among the stations. At Veli pH was 3.32, indicating the extremity of acidic factory effluents discharged from Travancore Titanium Product factory (TTP). This is in agreement with the observationsofearlier studies[40]. Relatively high DO (avg.5.04 ± 0.60) was observed at all stations except Veli. From these results, it is quite clear that the shelf waters of the Thiruvananthapuram coast have been exposed to the increased threat of industrial pollution.
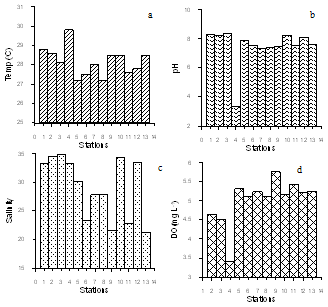 | Figure 2. bSpatial variation of hydrographic characteristics along the stations (a) water temperature (b) pH (c) Salinity(d) Dissolved Oxygen |
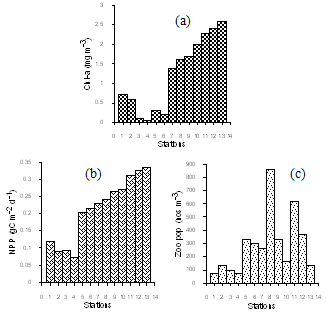 | Figure 3. Spatial variation of (a) pigment concentration (b) Primary productivity (c) zooplankton abundance |
 | Figure 4. Photomicrographs of some selected zooplankton recorded during the present study |
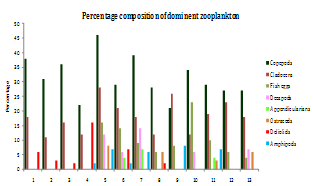 | Figure 5. Percentage composition of dominant groups of zooplankton in different stations |
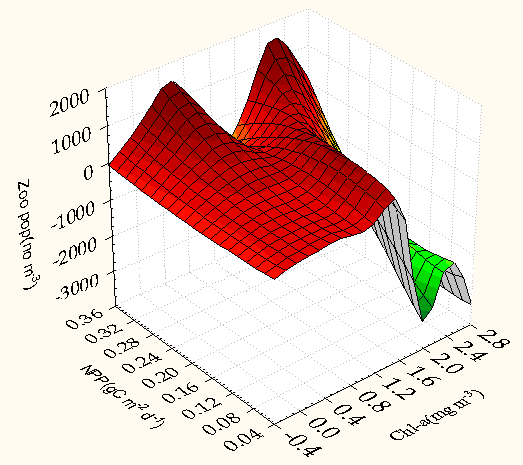 | Figure 6. 3D Surface plot of zooplankton population (no m-3) against chlorophylla (mg m-3) and NPP (g C m-3 d-1) zoo population (no m-3) = Distance weighted least squares |
|
4. Conclusions
- In the south west coast of India, presence and characterization of the sun screening compounds (MAAs) in the zooplankton community has been reported for the first time. Palythine and porphyra have been detected at all the stations studied. However, at four stations, mycosporine-glycine was present, where high pollution loads were there. Presence of this particular UV-absorbing compound, suggests that it is in response to pollution related environmental stress existing in these areas. High pollution loads in the stations have been reflected through the decreased levels of chlorophylla and of primary productivity. In addition to palythine, porphyra and mycosporine-glycine and some other unknown compounds were also present at Veli andHonavar. Quantification of MAAs of different zooplankton species can be performed to figure out their commercial application.
ACKNOWLEDGEMENTS
- Authors are thankful to the Ministry of Earth Sciences, Government of India for financial support during the study period. They also thank the Director, Regional Research Laboratory, Thiruvananthapuram, for encouragement. Deep appreciation to Mrs. M.Sindu, who rendered assistance in some of the experimental work. Thanks are also due to Dr.Oliver Nixdorf, Bremerhaven, Germany, for providing usstandards for the identification of MAAs.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML