-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Mining Engineering and Mineral Processing
p-ISSN: 2166-997X e-ISSN: 2166-9988
2017; 6(1): 20-24
doi:10.5923/j.mining.20170601.02

Modified Fatty Acids: Molecular Modeling and Docking Method for Optimization of Non-Sulfide Ore Flotation
P. M. Solozhenkin1, Sanda Krausz2, O. I. Ibragimova3
1Research Institute of Comprehensive Exploitation of Mineral Resources of the Russian Academy of Science (IPKON the RASci) Kryukovsky Tupik, Moscow, Russia
2University of Petrosani, Petrosani, Romania
3University of Dodoma, Dodoma, Tanzania
Correspondence to: O. I. Ibragimova, University of Dodoma, Dodoma, Tanzania.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Fatty acids are known as oxhydryl collectors which are used in practice for non-sulfide ore flotation. However, the selectivity of these collectors is low towards to gangue minerals occurring in the ore. In the present study the introduction of substitutes into the aliphatic chain of fatty acids has been suggested as the way for improvement the selectivity of collectors. Molecular modeling of alkaline earth mineral and modified fatty acid clusters has been performed using Chem Bio 3D and ChemOffice2005 by Cambridge Soft with optimization by MM2. DTF approach has been used to determine HOMO, LUMO and SOMO energies. The occurrence of the classical transfer of charge in bidentate complex with the decrease in charge on the calcium atom of the mineral cluster has been established. The strategy of prognosis of collector activity evaluation (PCAE) has been proposed as a consistent approach to estimate the interaction between a collector and a mineral cluster as a difference of total energy and sum of cluster energy and collector energy. The approach has proved useful in identifying relevant candidates for alkaline earth mineral flotation with modified fatty acids. The strengthening effect of binary mixture of modified fatty acids on flotability of alkaline earth mineralshas been established. To optimize the flotation the combination of oleic acid(OA) and ω - ((N,N-diethyl dithiocarbamato) undecanoic acid(DEDTCUA) has been investigated with the ratio of 1:1. By using this technique the fluorite concentrate has been upgraded up to 96,3% with the recovery of 85,35%.
Keywords: Modified fatty acids, Alkaline earth minerals, Molecular modeling, Collector activity evaluation, Flotation
Cite this paper: P. M. Solozhenkin, Sanda Krausz, O. I. Ibragimova, Modified Fatty Acids: Molecular Modeling and Docking Method for Optimization of Non-Sulfide Ore Flotation, International Journal of Mining Engineering and Mineral Processing , Vol. 6 No. 1, 2017, pp. 20-24. doi: 10.5923/j.mining.20170601.02.
Article Outline
1. Introduction
- Flotation reagent selection is paramount and test work is necessary to ensure that the optimum reagent regime is utilized. Oxhydryl collectors are principally used for flotation of oxidic minerals (silicates), carbonate materials and oxides. Fatty acids are considered to be widespread oxhydryl collectors for non-sulfide ore flotation. However, the selectivity of these collectors is low towards to gangue minerals occurring in the ore [1]. The introduction of substitutes into the aliphatic chain of fatty acids is one of the ways for improvement the selectivity of collectors. Although the connection between the spatial structure of molecules and the chemical activity of the compounds has been proven, the most troublesome question for researchers remains the estimation of quantitative structure-property relationship. Very little molecular modeling research has been carried out with alkaline earth minerals although these minerals are the most widespread in non-sulfide ores. Therefore, authors considered that this type of minerals has to be extensively studied. The present study is aimed at representing the computer modeling of fatty acids with long hydrocarbon chain and their derivatives in forecasting and selecting of prospective flotation reagents for minerals of alkaline earth metals. It is evident that the change in collector activity is correlated with the change in hydrophobicity [2-4]. It has been established that the fatty acid such as sulfopalmitic acid is the stronger and more selective collector for apatite and hematite flotation than normal palmitic acid [5]. Fatty acids can be also modified by substitution of halogen atoms in α – position in hydrocarbon chain resulting in strengthening of acidic properties and the greater ionization. This modification provides additional activity and the increase in collector adsorption on the mineral surface leading to the expansion of the optimum flotation of calcite and cassiterite, on the contrary, the flotation of wolframite has been shifted to more acidic area. It has been established that α –bromo palmitic acid possesses the higher collector activity than palmitic acid [6]. Early the number of sodium ω - (N, N – dialkyldithiocarbamato) undecanoates were identified as possible collectors for non-sulfide minerals [7]. The representatives of oxhydryl collectors with the general formula of R2N - C - S2 - (СН2) 10 – COONa that have been studied by physical-chemical methods are sodium ω - (N,N-diethyl dithiocarbamato) undecanoate (DEDTCU), sodium ω - (N, N- dibutyldithiocarbamato) undecanoate (DBDTCU).Modern computational methods and chemical programs allow to find the way of visualizing a three-dimensional model of the molecule and understand the connection between the spatial structure of the molecule and physical properties of the substance as well as its chemical activity for deeper understanding of these interrelations. Molecular modeling has been intensively developed over the past ten years. Significant research on computer modeling of oxhydryl reagents was carried out in India [8, 9]. The molecular structures of the various collectors were fully optimized in China using density functional theory (DFT) which offered an effective tool in the calculation of the properties and energies of the various collectors [10]. Molecular modeling of sulfhydryl reagents was carried out in Turkey [11], Finland [12] and Russia [13]. The knowledge generated in these studies can be extremely helpful in selection of collectors with desired properties as well as in designing of new reagents.
2. Methods and Objectives
2.1. Computational Methods
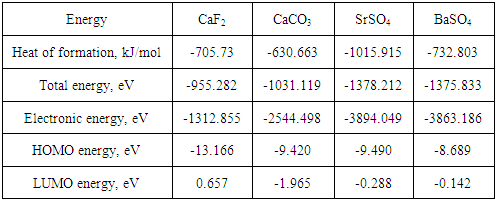
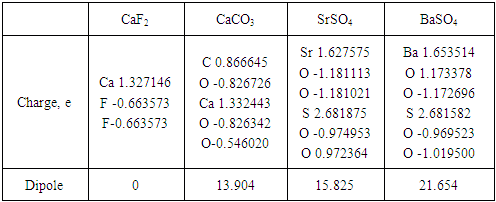
- Computational modeling of minerals and reagents was performed using Chem Bio 3D and ChemOffice2005 by Cambridge Soft with optimization by MM2. The semi empirical calculations were provided by MOPAC 2012 in vacuum [14, 15]. Molecular structures of mineral clusters were created. DTF approach was used to determine the optimal molecular structure and calculate atomic charge values, the compositions and energies of HOMO, LUMO and SOMO. The strategy of collector activity evaluation was proposed as a consistent approach to estimate the interaction between a collector and a mineral cluster as a difference of total energy and sum of cluster energy and collector energy [16]:
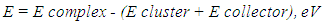 | (1) |
2.2. Flotation Tests
- Different samples of the ore were extracted and the flotation tests in laboratory and industrial flotation cells were conducted. The feed was conditioned with collectors followed by flotation operations.
2.3. Objectives
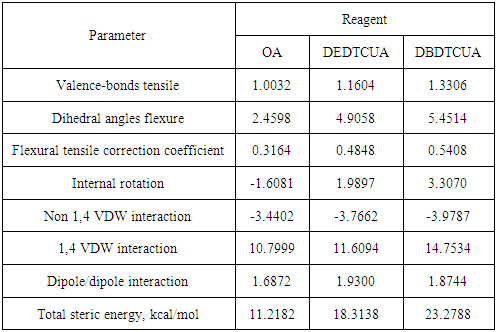
- The objectives of the present study were alkaline earth minerals, such as fluorite, calcite, barite and celestine. Oleic acid (OA) was used as a standard reagent to estimate the effectiveness of oxhydryl collectors studied. Oxhydryl modified collectors which could be prospective reagents for non-sulfide flotation, such as butylxanthatoundecanoic acid (BXUA), ω – (N, N-diethyl dithiocarbamato) undecanoic acid (DEDTCUA) and ω- (N, N- dibuthyldithiocarbamato) undecanoic acid (DBDTCUA), were proposed.
3. Results and Discussion
3.1. Molecular Models of Mineral Clusters
- Nowadays optimized geometrical models of various minerals of elements of platinum group, arsenic subgroup and sulfhydryl collectors, named mineral and reagents clusters, respectively, were created. The structures obtained correspond to chemical formulas as well as the distances between atoms correspond to tabulated data. In the present study the mineral clusters of alkaline earth minerals were created. Figures 1, 2 and 3 show the structural formulas (a) and 3D models (b) of alkaline earth mineral clusters.
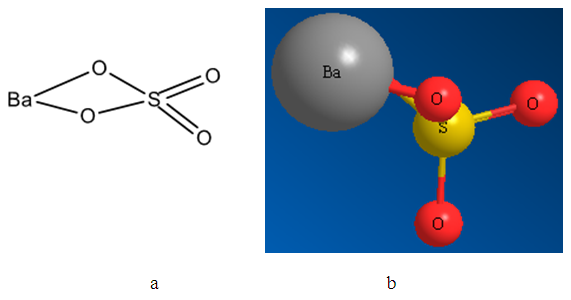 | Figure 1. Structural formula (a) and 3D optimized model (b) of the barite mineral cluster |
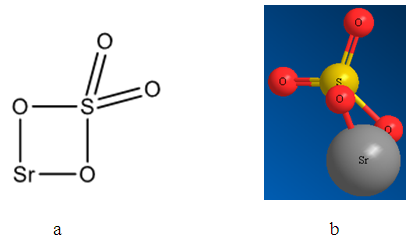 | Figure 2. Structural formula (a) and 3D optimized model (b) of the celestine mineral cluster |
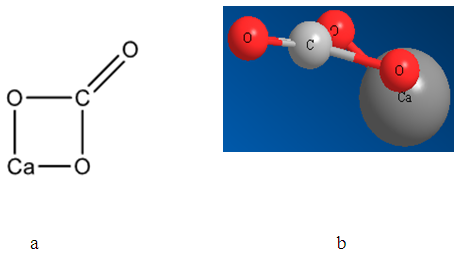 | Figure 3. Structural formula (a) and 3D optimized model (b) of the calcite mineral cluster |
|
|
3.2. Molecular Models of Flotation Reagents Clusters
- The modified fatty acids with the general formula R2N - C - S2 - (СН2)10 – COOH, where R – C2H5, C4H9, were studied and geometrical structures were created. The structure of BXUA is presented in Figure 4.
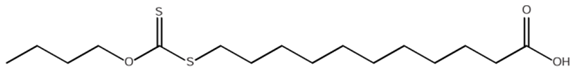 | Figure 4. Structural formula of butylxanthatoundecanoic acid (BXUA) |
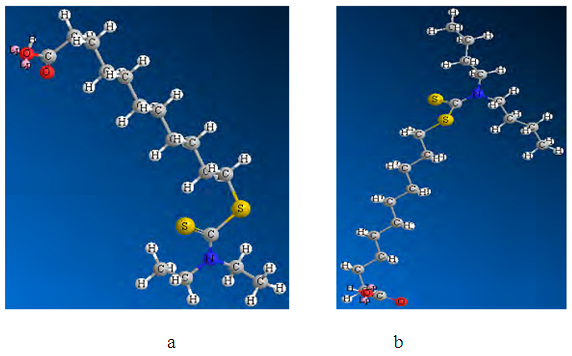 | Figure 5. Optimized 3D models of ω - ((N,N-diethyl dithiocarbamato) undecanoic acid (DEDTCUA) (a) and ω - (N, N- dibuthyldithiocarbamato) undecanoic acid (DBDTCUA) (b) |
|
3.3. Complex Formation and Prognosis of Collector Activity Evaluation (PCAE)
- The modified fatty acids attached to mineral clusters such as CaF2, CaCO3, SrSO4, BaSO4 were studied. Figure 6 shows BXUA attached to the fluorite mineral cluster as an example.
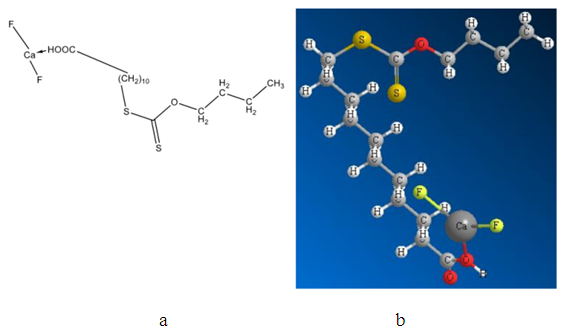 | Figure 6. Complex of the fluorite cluster with the molecule of butylxanthatoundecanoic acid (BXUA) attaching to the calcium atom (a) and its optimized geometrical model (b) |
3.4. Flotation of Alkaline Earth Minerals and Ores
- To optimize the flotation operation the combinations of oleic acid (OA) and ω – (N,N-diethyl dithiocarbamato) undecanoic acid (DEDTCUA) or ω - (N, N- dibuthyldithiocarbamato) undecanoic acid (DBDTCUA) were investigated with the ratio of 1:1. Application of the binary mixture of OA and DEDTCUA (1:1) allowed to increase the recovery of fluorite as well as the grade of the concentrate. The flotation was performed by using OA (100 g/t) and DEDTCUA (100 g/t) with the feed grade of 23,4% CaF2. By using this technique the fluorite concentrate was upgraded up to 96.3% with the recovery of 85.35%.
4. Conclusions
- Molecular modeling of alkaline earth mineral and modified fatty acid clusters was created. The main physical-chemical parameters were established. The transfer of electrons in the bidentate complex was investigated and occurred with the decrease in charge on the calcium atom of the mineral cluster.Index PCAE was calculated to analyze the docking activity of modified fatty acids with alkaline metals from mineral clusters. The approach was proved useful in identifying relevant candidates for alkaline earth mineral flotation with modified fatty acids. The strengthening effect of binary mixture of modified fatty acids on flotability of alkaline earth minerals was established. By application of the combinations of oleic acid (OA) and ω – (N,N-diethyl dithiocarbamato) undecanoic acid (DEDTCUA) or ω - (N, N- dibuthyldithiocarbamato) undecanoic acid (DBDTCUA) (1:1) the fluorite concentrate was upgraded up to 96,3% with the recovery of 85,35%.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML