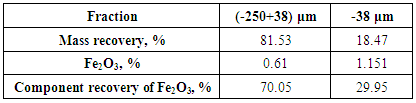-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Mining Engineering and Mineral Processing
p-ISSN: 2166-997X e-ISSN: 2166-9988
2016; 5(2): 25-34
doi:10.5923/j.mining.20160502.01

Reduce the Iron Content in Egyptian Feldspar Ore of Wadi Zirib for Industrial Applications
Ahmed M. M.1, Ibrahim G. A.1, Rizk A. M. E.1, Mahmoud N. A.2
1Professor at Mining and Metallurgical Engineering Department, Faculty of Engineering, Assiut University, Egypt
2Demonstrator at Mining and Metallurgical Engineering Department, Faculty of Engineering, Assiut University, Egypt
Correspondence to: Mahmoud N. A., Demonstrator at Mining and Metallurgical Engineering Department, Faculty of Engineering, Assiut University, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The presence of coloring materials such as iron oxides in feldspar decreases its quality. To use feldspar minerals in industry, some upgrading processes should be executed to remove impurities. The most important uses of feldspar are in ceramic and glasses. The present paper aimed to reduce the iron content in Wadi Zirib feldspar ore, as well as, to obtain an optimal grade of feldspar concentrate for some industrial applications. The first processing stage was the disposal of slime’s fraction (-38 µm) which contains clay minerals. Comminution and de-sliming processes removed about 30% of iron content into -38 μm fraction. The attrition process removed only about 6% of iron content. A dosage of 97 gm/ton of Quaternary ammonium salt solution was an optimum value for mica’s minerals flotation where the percentage of Fe2O3 was about 13.65% with mass recovery of 0.44% and component recovery of 9.84%. The rejected percentage of valuable minerals into the floated mica’s minerals didn’t exceed 0.5%. A flotation test was carried out at optimum conditions for flotation of feldspar minerals. The mass recovery of feldspar concentrate was 52.11% of feed (-250+38) µm. At such optimum conditions, a suitable feldspar concentrate was obtained with 0.4% Fe2O3. The component recovery of iron content removed into feldspar tailing was about 56%. The specifications of feldspar concentrate obtained in this research fulfilled the requirements of some industries, i.e. glass, ceramic vitreous tiles, and semi vitreous tiles. The final results revealed that the total disposal percent of iron content was about 75% of that present into the feed head sample.
Keywords: Flotation, Feldspar, Mica’s minerals, Ceramics, Glass
Cite this paper: Ahmed M. M., Ibrahim G. A., Rizk A. M. E., Mahmoud N. A., Reduce the Iron Content in Egyptian Feldspar Ore of Wadi Zirib for Industrial Applications, International Journal of Mining Engineering and Mineral Processing , Vol. 5 No. 2, 2016, pp. 25-34. doi: 10.5923/j.mining.20160502.01.
Article Outline
1. Introduction
- Feldspar is one of the most common minerals in the world where it forms about 60% of the rocks of the earth's crust [1-6]. Orthoclase (K-feldspar), albite (Na-feldspar) and anorthite (Ca-feldspar) are the most widespread feldspar minerals. The most associated minerals into feldspar ore are clays, mica’s minerals (i.e. biotite and muscovite), tourmaline, rutile, and sphene [6-10]. The presence of coloring materials such as iron oxides and rutile decreases the quality due to forming a black spot in the product body during firing process [10]. To use feldspar minerals for different industrial applications, some upgrading processes should be executed to remove impurities [11].Feldspar mineral intervenes in the ceramic and glass industries. Seventy percent of feldspar minerals productions in the world are used in manufacture of glass products, i.e. insulation fiberglass [12]. The rest is used in ceramic products and other applications such as fillers and extenders in plastics, paints, rubber, welding electrode, polymer, paper, and paint industries [4-5, 11].The most efficient method for upgrading and removing the coloring materials from feldspars is magnetic separation. A High intensity magnetic separator is employed for the low iron content ores. Flotation is the most method that can handle many feldspar ores with different iron contents [8, 11].Generally, the first processing stage of feldspar ore is to get rid of slimes which is usually clay minerals [9]. Slime fraction is about -38 µm which produces larger surface areas and consequently causes excess in collector consumption [13]. Feldspar can be separated from the associated minerals by using multi-stage flotation processes.Quaternary ammonium salt and dodecylamine can be used as mica’s mineral cationic collectors [14]. All mica’s minerals can be readily floated in an acidic medium around a pH of 2 with a cationic collector at a dosage of about 0.1-0.5 kg/ton. Under these conditions, neither quartz nor feldspar will float unless activated [3, 15]. Feldspars are floated from quartz using primary long-chain alkyl amine cationic collectors at acidic medium [4].Abdel-Khaled et. al. [16] used quaternary ammonium salt as a cationic collector for feldspar flotation. Sekulic et. al. [17] compared between different types of cationic collectors like Flotigam DAT, Armoflot 64, and Aero 3030C for flotation of feldspar and mica. They revealed that Aero 3030C was more selective and preferred in feldspar and mica’s mineral flotation. The present paper aimed to reduce the iron contents of Wadi Zirib feldspar ore and also to obtain an optimal commercial grade of feldspar which is convenient for some industrial applications.
2. Experimental Work
2.1. Geology
- The barrier of Wadi Zirib feldspar ore is considered the intersection of Wadi Al-Asyud with Wadi Bayda Al-Atshan which is located at the Eastern Desert, Egypt. The entrance of Wadi Zirib is 15 Km away from qusier on qusier Marsa Alam paved road. Wadi Zirib area lies between latitudes 25° 59' and 25° 01' N and longitudes 34° 11' and 34° 14' E as shown in Fig. 1 [18]. This region extends from north to south for about 10 Km. The topography of this area is mostly granitic rocks containing feldspar minerals. The average percentages of K2O and Na2O are about 4.5 and 4.0%, respectively. The basement rocks are of Precambrian age. They are classified into four outcrops. The youngest one is granite, the younger is gabbro, the older is granitoids and the oldest one is meta-volcanic. The emplacement of the granitic pluton is followed by injection of dykes and veins of different shapes and composition [18].
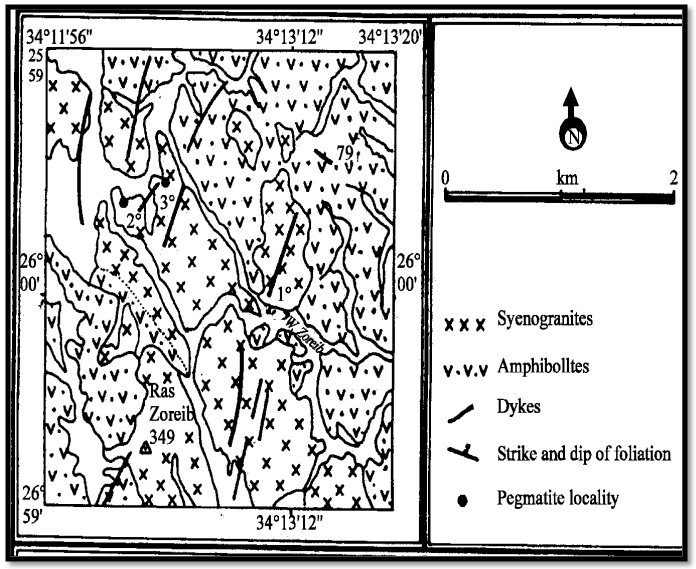 | Figure 1. A geological map of Wadi Zirib area, Eastern Desert, Egypt [18] |
2.2. Procedure
- The sample used in the present work was crushed to -10 mm in a laboratory jaw crusher then to -5 mm in a small jaw crusher. The -5 mm sample was crushed to -2 mm using a roll crusher in a closed circuit with 2 mm sieve. The roll crusher product was ground to -250 μm using a porcelain mill in a closed circuit with 250 μm sieve. The slimes (-38) μm was separated by wet screening.An attrition process was carried out on the fraction (-250+38) μm in an attrition apparatus. The attrition process was executed at optimum conditions with solids percent of 65% and 1430 rpm impeller speed for 15 min. The attrition product was wet sieved on a screen of 38 μm.The fraction (-250+38) μm, obtained from comminution process, was used for mica’s minerals flotation experiments. A flow sheet of comminution and attrition processes of feldspar sample was illustrated in Fig. 2.
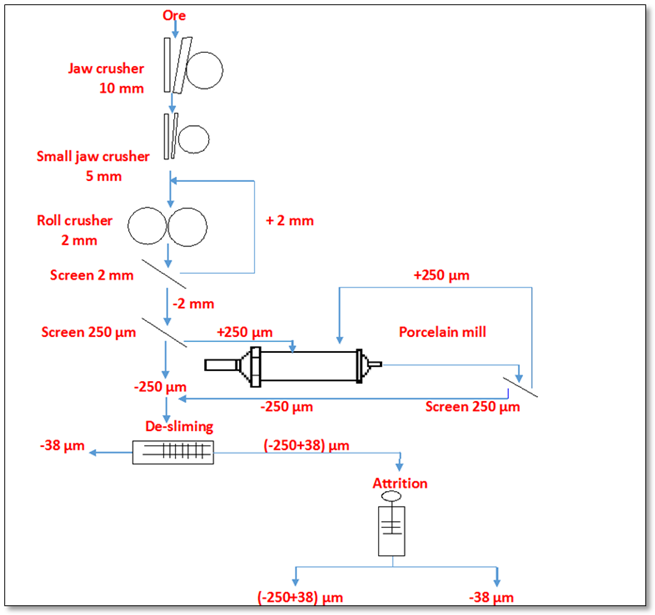 | Figure 2. Flow sheet of comminution and attrition processes of head sample |
3. Results and Discussions
3.1. Mineralogical Description
- The microscopic study of the samples illustrated that the rock containing feldspar minerals is holocrystalline, hypidiomorphic with equigranular textures. They are composed mainly of potash feldspar, quartz and plagioclase in variable proportions and minor amounts of muscovite, biotite, and rare opaque minerals. Plagioclase crystals are coarse to medium grained. Sometimes the outlines of plagioclase phenocrystals are corroded by quartz having graphic texture as shown in Fig. 3. Fresh plagioclase crystals showed albite and pericline twining. They were partially to completely altered to sericite and clay minerals as illustrated in Fig. 4.
 | Figure 3. Photomicrograph showing graphic texture (25 X, C.N) |
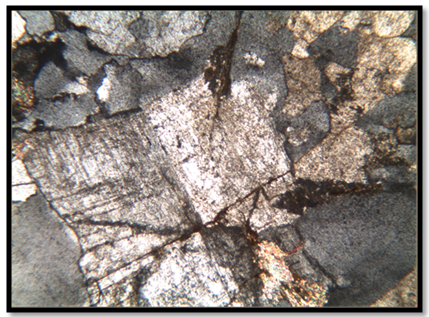 | Figure 4. Photomicrograph showing plagioclase cracked which partially altered to sericite and clay minerals (25 X, C.N) |
 | Figure 5. Photomicrograph showing biotite partially altered to iron oxides, muscovite and chlorite (25 X, C.N) |
 | Figure 6. Photomicrograph showing micro fracture partially filled with opaque minerals which cutting across essential mineral constituents and takes nearly the same directions (25 X, C.N) |
3.2. Sample Analysis
- The chemical composition of feed sample was obtained by XRF analysis as follows: 75.1% SiO2, 14.0% Al2O3, 0.07% TiO2, 0.71% Fe2O3, 4.0% Na2O, 4.5% K2O, 0.16% MgO, 0.61% CaO and 0.46 L.O.I. The results of XRD analysis revealed that the main minerals in feldspar ore are quartz, albite, and microcline and the secondary minerals are mica’s minerals (i.e. muscovite, biotite, chlorite, rare opaque minerals, and iron oxides) as illustrated in Fig. 7.
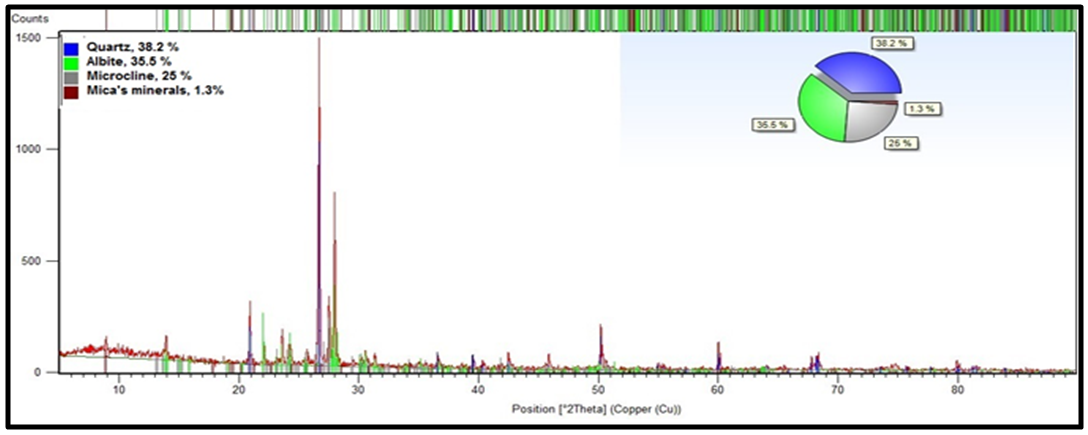 | Figure 7. XRD results |
3.3. Method Description
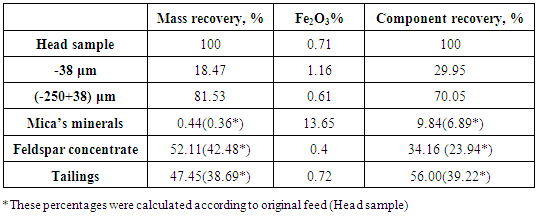
- The present work explained the possibility of reducing the percentage of iron content into the feldspar ore to be suitable for industrial applications. Figures 8 and 9 showed the final results of preparation the head sample to flotation process. These figures included flowcharts of the comminution and de-sliming processes of the head sample. The results showed that these processes reduced the percentage of Fe2O3 from 0.71% into the head sample to 0.61% into (-250+38) μm fraction with a mass recovery of 81.53%. The component recovery of Fe2O3 was about 70%. The final results are illustrated in Table 1. The obtained results revealed that comminution and de-sliming processes removed about 30% of iron content into fraction -38 μm. The diminution of Fe2O3 by comminution processes suggests that iron is not completed to feldspars and should be related to Fe-associated minerals. The comminution process liberated the associated minerals that contain part of iron from the feldspar.
|
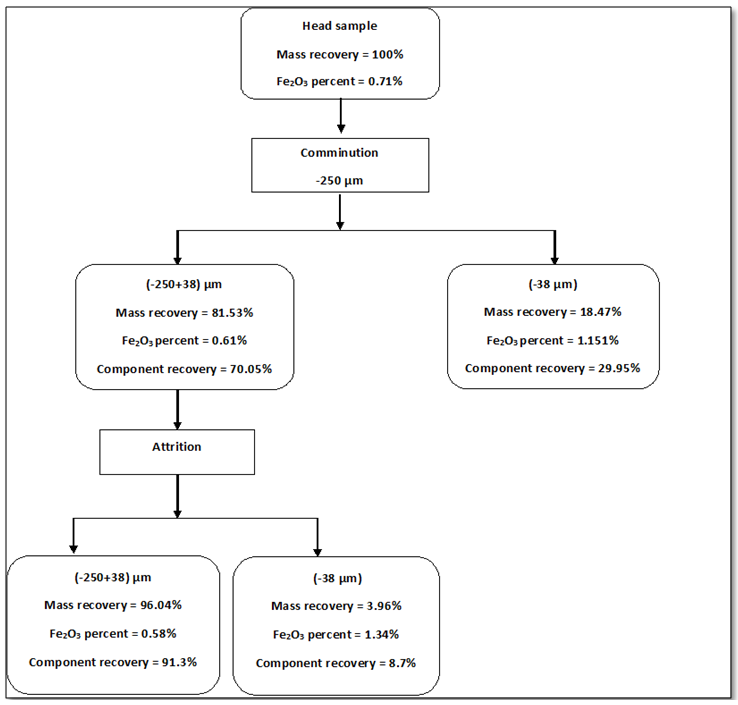 | Figure 8. Flow sheet of the results of comminution and de-sliming processes of head sample |
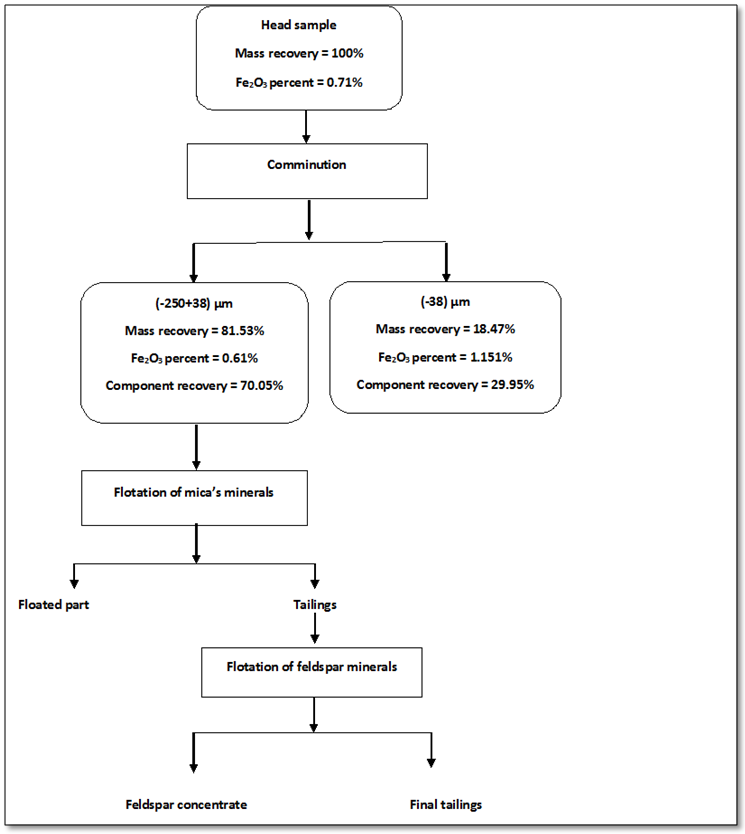 | Figure 9. The final results of head sample to flotation process |
|
|
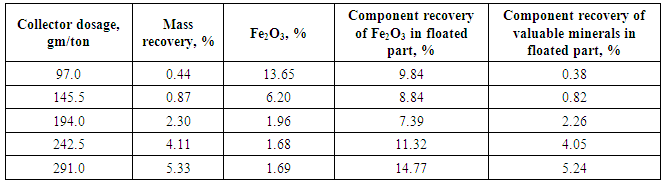
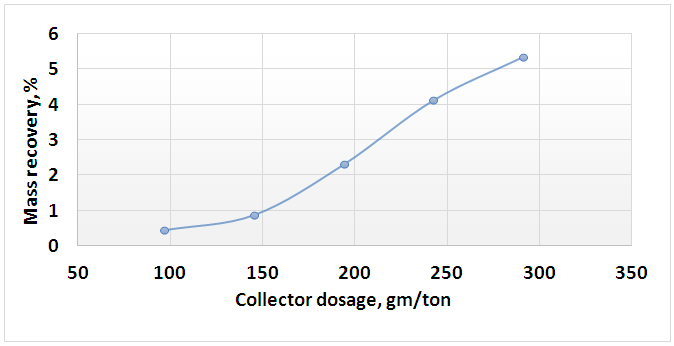 | Figure 10. Effect of collector dosage on mass recovery of floated mica’s minerals |
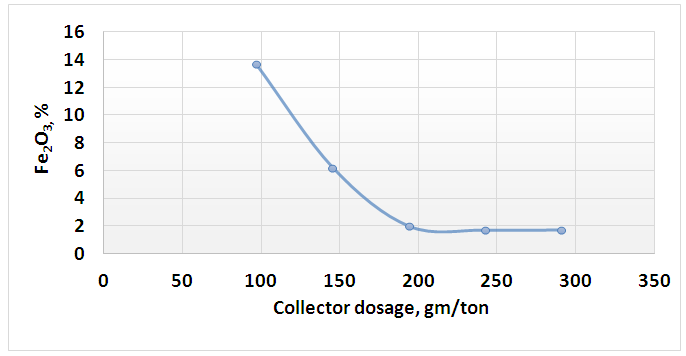 | Figure 11. Effect of collector dosage on percent of Fe2O3 in floated mica’s minerals |
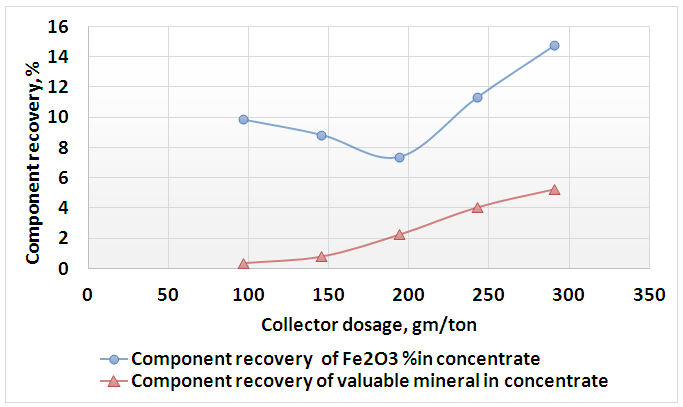 | Figure 12. Effect of collector dosage on component recovery of mica’s minerals and valuable minerals into floated part |
|
|
4. Conclusions
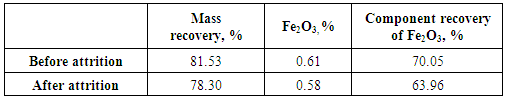
- 1. The comminution and de-sliming processes reduced the percentage of Fe2O3 from 0.71% into the head sample to 0.61% into (-250+38) μm fraction with a mass recovery of 81.53%. The component recovery of Fe2O3 content was about 70%. These processes removed about 30% of iron content into fraction of -38 μm.2. The attrition process decreased the percent of Fe2O3 from 0.61% to 0.58%. This process removed only 6.09% of the iron content into fraction of -38 μm.3. a dosage of 97 gm/ton of quaternary ammonium salt solution is an optimum for mica’s minerals flotation where the percentage of Fe2O3 was the maximam one (13.65%).4. A feldspar concentrate with 0.4% Fe2O3 and mass recovery of 52.11% were obtained. The component recovery of iron content removed into the tailings of feldspar flotation was about 56%.5. Feldspar concentrate specifications obtained in this reserch fulfilled the requirements for glass, ceramic, vitreous tiles, and semi vitreous tiles industries.6. The total iron content removed into the final tailings represented about 75% of the total iron present into original feed sample.
ACKNOWLEDGMENTS
- The authors would gratefully thank Dr. Hassan Bekhit Abdel Rahman. He is an authority-Chairman of Arab Geologists Association Egyptian Mineral resources. We thank him so much for his assistance to make the mineralogical study of samples and for his comments that greatly improved the present research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML