Ajibola Olawale Olarewaju 1, Boluwade Edward Ayodele 2, Borisade Sunday Gbenga 1
1Department of Materials and Metallurgical Engineering, Federal University Oye Ekiti, Oye Ekiti, Nigeria
2Department of Minerals and Petroleum Resources Engineering Tech., Federal Polytechnic, Ado Ekiti, Nigeria
Correspondence to: Ajibola Olawale Olarewaju , Department of Materials and Metallurgical Engineering, Federal University Oye Ekiti, Oye Ekiti, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Abstract
This work reports the result of a mineralogical investigation of the Itapa Ekiti rock samples. Samples were collected from six locations within the town. The physical properties (colour, streak, and hardness) of their mineral contents were evaluated. The crushing strength was determined and the chemical analysis by Atomic absorption spectrometry techniques was used. The petrologic analysis shows that the rock is highly mineralised. The results revealed about seventeen metals are present in the rock samples which are mostly dominated by alkali and alkali earth metals such as 7.4% Na, 5.8% K, 7.6% Mg and 6.5% Ca with other heavy metal associates like 5.1% Zn, 5.4% Fe, 5.1% Cu and 2.7% Ni. This provides useful information on the Itapa Ekiti rocks for the exploration and exploitation of these vast industrial minerals.
Keywords:
Mineralogy, Itapa Ekiti, Feldspathic-Biotite Rock, Mineral Processing
Cite this paper: Ajibola Olawale Olarewaju , Boluwade Edward Ayodele , Borisade Sunday Gbenga , Geometallurgical Evaluation of Itapa Ekiti Feldspathic-Biotite Ore Deposit for Effective Processing and Extraction, International Journal of Mining Engineering and Mineral Processing , Vol. 4 No. 1, 2015, pp. 1-7. doi: 10.5923/j.mining.20150401.01.
1. Introduction
The availability of sufficient information of the geology, mineralogy of ore and ore texture assist in the effective design of concentration flow-sheet. More importantly, these characteristics facilitate setting the proper grinding size to get required liberation and reduce over-grinding, and help out in discovering correct concentration (flotation) constraints to achieve the optimum mineral separation [1]. A vast number of reports of research work had been published over the years on the geological exploration [2, 3], mining survey [4, 5] and metallurgical exploitation (mineral processing and extraction) [6-11] of numerous mineral deposits discovered across the boundaries of Nigeria. The major focuses of such reports were to develop theses mineral for economic applications [12].Ekiti being one of the developing states among the 36 states in Nigeria, presently involved and striving in the establishment of various manufacturing and construction industries some of which may require the use of very large quantity of many industrial minerals and rocks present in the geographic area. Ekiti state is covered with vegetation and rocks that has been formed through the cooling solidification of magma which might have under gone the processes of metamorphic rock formation [13].Itapa Ekiti is one of the many towns located in Oye Local Government of the Ekiti state on the Lat 7o, 48'N and Long 5o, 23'E (Figure 1). It is surrounded by rocks that are predominantly granite and believed to have been exposed through several years of erosion activities. Geographically, the terrain of Itapa Ekiti and its environs belong to complex basement that are characteristically metaigneous [14]. Literatures are scarcely available on the record of research works done on the economic minerals present in Itapa Ekiti. Also the quest to search for more industrial minerals in Ekiti state have daily motivated the industrial mineral engineers and geoscientist in finding out some of the economic minerals present in Itapa Ekiti rocks.Intrusive igneous rocks form very good engineering materials, possessing high crushing strength and shearing strength especially when they are fresh and un-weathered, unless they are minutely fractured. They make a good source of concrete aggregate and typical construction materials. Paying much attention to jointing and possible faulting, weathering along fractures, some tests may be necessary before using it for engineering construction. On the other hand, the engineering properties of sedimentary rocks depend largely on the degree of compaction, consolidation and cementation, these, which determine the hardness, crushing strength and shearing resistance (Strength) of the rocks. Metamorphic rocks share structural complexity in both physical and chemical properties and hence, make it impossible to generalize their engineering properties.It is necessary to assess the suitability of the Itapa rock through geochemical and petrographic study for the abundance of the Precambrian ore in Ekiti State, Nigeria and for their economic potential in building construction and extractive industry.
2. Methodology
2.1. Materials Sampling
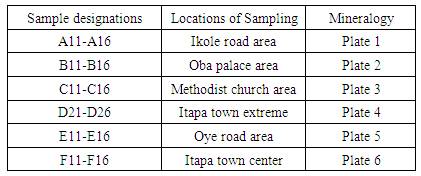
A total of 36 rock samples were collected from six (6) different locations within Itapa Ekiti and the samples were labelled A11-A16, B11-B16, C11-C16, D21-D26, E11-E16 and F11-F16.
2.2. Equipment and Materials
Some of the equipment used for the experiment include: Denver jaw crusher, pulverizer, (vibrating mill), sieve shaker, streak plate and hardness test (durometer), hydraulic compressive strength tester, Petrologic photo-microscope and Atomic Absorption spectrometer (AAS). Standard grades of HCl, HF, HNO3 Boric acid and lanthanium chloride were all purchased from the chemical store.
2.3. Experimental Procedure
(A) Physical Properties DeterminationThe following physical examinations were carried out on the six (6) samples (A, B, C, D, E and F) of Itapa-Ekiti rocks. Each of the properties carried out include: colour, lustre, streak hardness, crushing strength, cleavage, texture, and the results were presented in Table 1.(i.) Colour: Some mineral can be easily identified just from their colour. The variation of colour in most minerals is due to atomic substitution or presence of impurities. Colour alone is not usually sufficient to identify and distinguish a mineral. (ii.) Lustre: This is the behaviour of the minerals to reflection or absorption of light incident on them which gives it a characteristic appearance. The mineral which will allow the passage of light through it or the edges are said to have non-metallic lustre and are further described as: vitreous or glassy, resinous, silky, pearly, adamonite and waxy or greasy(iii.) Streak: This is the colour that is obtained when a mineral is grinded to powder. It is different from the initial and usual colour of the mineral itself. The mineral is rubbed across a streak plate, and will leave a trail of powder on the plate.(iv.) Hardness test: The hardness tests of samples are determined using Brinell Hardness Testing Machine and the values are calculated from equation (1)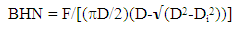 | (1) |
where BHN = the Brinell hardness numberF = imposed load in kg D = diameter of the spherical indenter in mm Di = diameter of the resulting indenter impression in mm (v.) Crushing strength: It is carried out by placing the specimen on a flat surface followed by application of a uniform load to it through a bearing block in a standard mechanical or hydraulic compression testing machine. The load at which cracks appear in the refractory specimen represents the CCS of the specimen. It is determined as (2)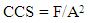 | (2) |
where F = Applied load, A= Cross sectional area of specimen(vi.) Cleavage: Mineral shows cleavage when it breaks along smooth surfaces in definite directions. The surfaces can be easily seen due to the reflection of light. Cleavage is usually described as perfect, good, fair or poor together with the direction of cleavage.(B) Leaching of Rock SamplesEach of the six rock samples were crushed and milled to fine particles using the laboratory size jaw crusher and the vibrating mill. The grinded samples were sieved to obtain -63μm particle size. 1.0g of each of the samples A, B, C, D, E and F were weighed into a 100 ml plastic container in which 5 ml HF was added. 10 ml of aqua-regia (HNO3) was also added. The containers and their contents were heated up on a stream boiler for 45 minutes. 5 ml saturated solution of boric acid was then added to each of the boiled rock solutions. After, the samples were made up to the mark with distilled water (100 ml) and lanthanum chloride solution was added to prevent interferences during AAS analysis. The AAS analysis calibration stock standard was taken to be 10 ppm for each of metals analyzed. For the instrumentation, a bulk model 200AA flame system was used, air – acetylene were used for all metals. Normal instrumental parameters were employed for all the analysis. Equation (3) was used to determine the AAS parameters of the rock samples and the results were presented in Table 2.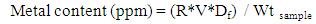 | (3) |
where R= Sample reading /Standard reading, V = Vol. of solution = 100 ml,Df = Dilution factor = 10.Wt sample = Weight of sample
2.4. Particle Size Analysis
About 500g of the sample was weighed, crushed and grinded using the Denver jaw crusher and vibrating mill (Figure 2 a, b). This test involves passing a (500g) representative crushed sample through a set of sieves (4750 to 63 µm aperture) arranged in descending order with the largest sieve size at the top. The sieves were clamped on the sieve shaker for about 15 minutes. The sieves were later removed and reweighed with their corresponding contents. The undersize crushed particle size range was selected between (-212 to -63) while the oversize (+425 to +212 µm) was take for secondary reduction (grinding). In each of the comminution stage the he quantity of weight retained (% R) in each screen is determined as in (4):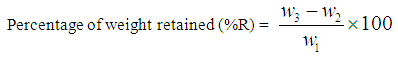 | (4) |
where W1 = weight of sample usedW2 = weight of empty sieveW3 = weight of sieve + sampleW3 – W2 = weight of sample retained.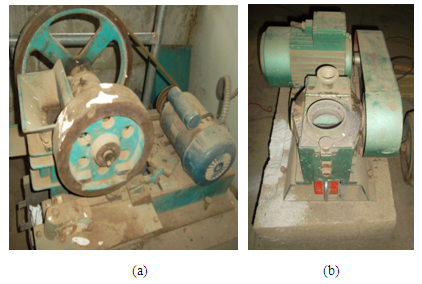 | Figure 2. Crushing (a) and (b) grinding equipments |
3. Results and Discussion
The results of the physical properties of the Itapa Ekiti rock samples revealed the six rock samples have very close physical characteristics.Table 1 shows that the samples collected from different locations within deposit in the township have an average hardness value of 6.75 on the Rockwell scale. This value is very close to a generally accepted hardness value for quartz (7.0) and olivine (6.5-7.0) as reported by Burchefield [15]. The colour varies from brownish red to dark brown. A typical rock may contain alternating lighter layer (leuocosome) such as feldspar and quartz in combination of dark layer (melanosome) such as biotite [3, 16]. The mineral lustre is glassy while the streak varies from whitish to buff colour. A fairly high crushing strength (kN/mm2) ranging from 49.32 to 50.19 (kN/mm2). The cleavage varies from low faint to faint while the texture varies from fine to medium. The fracture patterns observed are defined by parallel and sheets characteristics.Table 1. Mineral Physical Identification Properties (MPIP) of Itapa rock samples
 |
| |
|
The investigated rock specimens in Table 1 could be relatively compared with any known igneous rock or rather an hybrid metamorphic igneous rock [17, 18] It is known and established that igneous rocks are made up of silicate minerals, and often in crystalline forms of coarse or medium grain size like quartz and feldspar [3]. Quartz identified as greyish glassy looking, harder than steel while feldspar present in igneous rocks are orthoclase, having pink to white crystals. According to Carlos et al, [19], rich occurrences of quartz and feldspar may indicate that leucosome is present in such selected rock. The plagioclase is greyish white both are harder than steel and show low faint cleavage planes according to Raymond [20]. It has been established that plagioclase are common in granitic pegmatite and the granite stuffs according to Berry, [21]. The geochemical analysis some selected outcrop of Itapa Ekiti rock samples are presented in Table 2. The average values (%) of seventeen major metals identified in the samples signify the richness of metallic minerals present in the samples. Large scale rich deposits of Pb-Zn sulphide complex ore and Iron ore have been for long discovered and explored in the country [11, 12]. In the same vein, the Itapa Ekiti rocks is containing very high amount of Pb, Zn, Fe, Cr, Cu, Al and Ni containing minerals beside the alkali metals (Mg, Ca, K, Na) in the sample.Table 2. Geochemical analysis of Itapa Ekiti rock samples
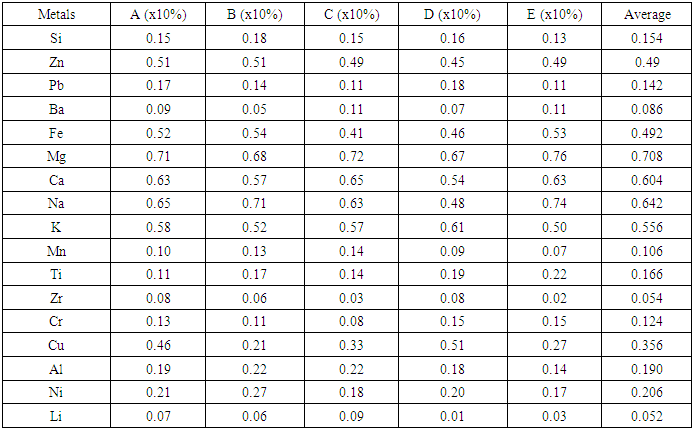 |
| |
|
Apart from the available information on mineralogy of ore, the ore texture helps out in the effective design of separation flow-sheet. For an efficient metallurgical exploitation of any mineral proper particle sizing are required in the liberation and in reducing over-grinding.These as well help out in discovering correct concentration (flotation) control parameters required to optimise the mineral separation. The particle size distribution of crushed rock sample is presented in Table 3. It is obvious that the liberation posed no difficulty despite the average hardness value of 6.75 and fairly high crushing strength (49.32 - 50.19 kN/mm2).Table 3. Particle size (µm) distribution of crushed rock sample
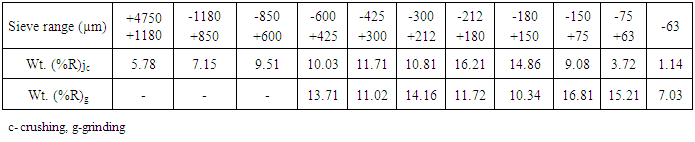 |
| |
|
The sieve analysis in Table 3 shows that not less than 45% (225.05 g) of the crushed rock particles reported as undersize (-212 to -63 µm) in a single stream of size reduction using a laboratory size Denver jaw crusher.From about 32.55% (162.75 g) of the size -600 to +212 µm returned for grinding in the vibrating mill, 61.11% (99.46 g) was reported as undersize (-212 to -63 µm) in a single stream of size reduction (grinding). Egunlae and Oluwaseyi [22] as well as Ajibola et al. [11] had earlier reported the successful flotation of -212 ~ +75 µm PbS/Zns particles. Hence there is much prospect in the comminution of these minerals for flotation and extraction purposes.
3.1. Analyses of Rock Sample
The petrographs and the mineral contents of selected rock samples are reflected in Plates 1-6 and in Table 4. The photo-petrologic of the mineral thin sections are examined as the cross polar and plane polar views.Table 4. Mineral contents of selected rock samples
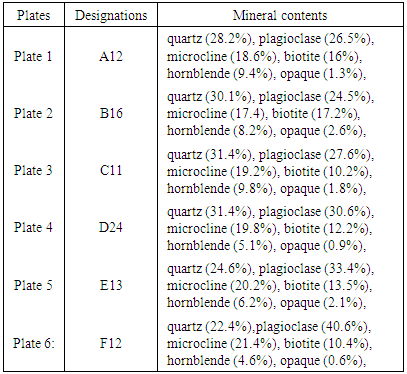 |
| |
|
 | Plate 1. Petrography of Itapa Ekiti rock (Sample A12) x80 mag |
 | Plate 2. Petrography of Itapa Ekiti rock (Sample B16) x80 mag |
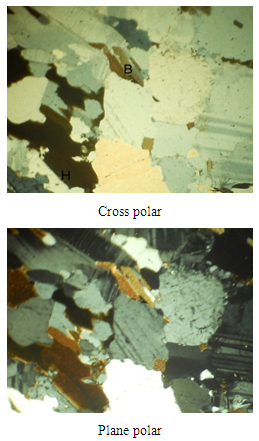 | Plate 3. Petrography of Itapa Ekiti rock (Sample C11) x80 mag |
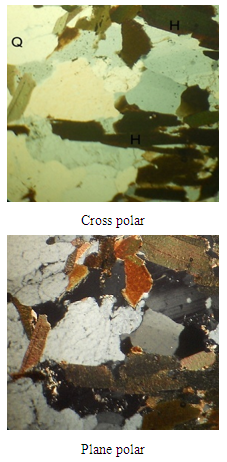 | Plate 4. Petrography of Itapa Ekiti rock (Sample D24) x80 mag |
 | Plate 5. Petrography of Itapa Ekiti rock (Sample E13) x80 mag |
 | Plate 6. Petrography of Itapa Ekiti rock (Sample F12) x80 mag |
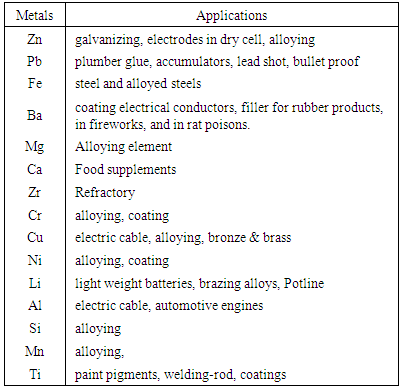
The combination of the petrographs and the geochemical analysis show that the rock is highly mineralised. Different types of minerals such as quartz, plagioclase, microcline, biotite, hornblende and opaque minerals are found in the deposit. As observed in the petrographs (Plates 1-6) and in the mineral contents of selected rock samples in Table 4, the presence of opaque minerals suggests the presence of metallic minerals in the deposit.From Table 2, the results of geochemical analysis revealed that about seventeen metals were identified to be present in large quantities within the ore body. The ore is primarily dominated by the alkali and alkali earth metals such as Na, K, Mg and Ca with about 0.65%, 0.58%, 0.71% and 0.63% content respectively. Apart from the alkali metals, the ore (rocks) could be assessed to be rich in-other metals having 0.51% Zn, 0.52% Fe, 0.46% Cu and 0.21% Ni. The vast number and occurrences of other metals in smaller quantities within the rocks samples could be explained from the understanding of rock metamorphism. For metamorphic rocks, heat and pressure may cause the chemical elements in the original rock to react and reform into new minerals. In this process, no elements are added to or taken away from the original elements simply re-arranged to suit new conditions [3, 19, 21]. Although metamorphism may involve great heat, the rock does not melt as it changes [15]. Hence, the high percentage of alkali and alkali earth metals like Na, K, Mg, Ca, Ba, Si, Fe and Li present in the ore (Table 2) points to the fact that such igneous rock is composed largely of quartz and alkali feldspar.Apart from using the rocks in engineering constructions works, the occurrence of a large number of heavy and transition metals such as Ti, Cr, Mn, Fe, Ni, Cu, Zn, and Al shows that the rocks samples are rich in other economic minerals from which these valuable metals could be won for industrial use.Lead is found as Galena PbS, (regularly with sphalerite, chalcopyrite, pyrite, tetrahedrite, and gangue minerals such as calcite, quartz. It is also in pegmatites, and in the company of garnets, rhodonite, feldspar, diopside and biotite. Copper in most cases occurs as Chalcopyrite CuFeS, Bornite Cu5FeS4, Chalcocite Cu2S, Covellite CuS, (but most often in veins in the midst of other sulphides, such as galena, sphalerite, pyrrhotite, pyrite, and also cassiterite, common gangue minerals quartz, calcite, dolomite. disseminated amid bornite and pyrite in porphyry copper deposits). Some principal minerals of Nickel are pentlandite (FeNi)S, garnierite (hydrated Ni-Mg silicate) and millerite NiS with other nickel minerals and sulphides. Zinc minerals occur as Sphalerite ZnS, (associated with pyrrhotite, pyrite and magnetite), Hemimorphite (Calamine) Zn4Si2O7.(OH)2.H2O (the sulphides of zinc, iron, and lead), Smithsonite ZnCO3 (sphalerite, galena, and calcite) Marmatite (Zn, Fe)S (associate with galena). Some engineering applications of some of the metal values that could be explored economically are shown in Table 5.Table 5. Some Engineering Applications of the Metal Values
 |
| |
|
4. Conclusions
Petrologic analysis shows that the rock is highly mineralised. The geochemical mineral analysis revealed that seventeen metals were identified. This work provides useful and meaningful information on the presence of valuable metals in Itapa Ekiti rocks. It also draws the awareness of the government and other investors to this locality for the commencement of exploration and exploitation of these vast industrial minerals and metals available in Ekiti State, Nigeria. The rocks are granitic, useful for construction materials such as crushed rock, polished rocks and aggregates. Since the recycling of metals from discarded scraps cannot meet the demand for metals and alloys needed in Nigeria, more efforts should be drawn toward boosting the production preferably by hydrometallurgical route and electrometallurgical processes. Studies are still on going on the process design for the cost effectiveness of the whole metallurgical processes in order to validate the economic profitability of the processing for free enterprise development.
ACKNOWLEDGEMENTS
The authors express their sincere gratitude to the management of the Minerals and Petroleum Resources Engineering Technology Dept., Federal Polytechnic. Ado Ekiti for providing the workshop and laboratory for the works.
References
| [1] | K. Tungpalan, E. Wightman, E. Manlapig, Minerals Engineering.,03/2015; DOI: 10.1016/j.mineng.2015.02.005 |
| [2] | E.A Fayose, Federal University of Technology, Akure Annual Lecture Series, 1987, (5). |
| [3] | E.A Bolowade and C.O Ayodele, In: F.I Aluko (Ed), Proceedings of the 9th Engineering Forum, 14-16 August 2014, Ado Ekiti, Nigeria, 2015, (2), 33-46. |
| [4] | J.M Akande and D.A Awojobi, Journal of Engineering and Technology. 2003, 10 (2) 4891-4900. |
| [5] | B.O Jimoh, P.O. Obisesan, L.A Azzez., Journal of Engineering and Earth Sciences, 2007, 2, (1): 67 – 72. |
| [6] | O.A.Fasuba, O.O Egunlae, B.O Jimoh. Journal of Engineering Technology and Industrial Applications. 2002, 1(2): 72-75. |
| [7] | O.O Egunlae, P.O Obisesan, A.O Adeloye. Nigeria Journal of Engineering Management, 2006, 7(4): 46-52. |
| [8] | O.O Egunlae, A.O Adeloye, D.T Oloruntoba, Journal of Engineering and Earth Sciences, 2006, 1(1): 57-65. |
| [9] | A.O Oluwaseyi, O.O Egunlae. Journal of Engineering and Earth Sciences. 2007, 2(1): 6-11. |
| [10] | O.O Ajibola, B.O Jimoh. Advances in Applied Science Research, 2014, 5(3):68-72. |
| [11] | O.O Ajibola, D.T Oloruntoba, A.F Owa. In K. Ulrich. The Proceedings of Lead-Zinc Conference Pb-Zn 2015, June 14-17 2015, Dusseldorf, Germany. 2015(2): 561-575. |
| [12] | O.O Onyemaobi. Proceedings of the Nigeria Society of Engineers National Conference and Annual General Meetings. Nov 5-9, 2001. Port Harcourt, Nigeria, pp 21-25. |
| [13] | M.A Oyawoye (2001): Geology of basement complex. Journal of Nigeria Mining, Geology and Metallurgy. Vol 1. pp 88-102. |
| [14] | S. Cooray, M.A. Rahaman, Review of basement complex of Southwestern Nigeria. University press. Ibadan, Nigeria, 2004, pp331-343. |
| [15] | C.B Burchefield, Physical Geology. Merill Pub. Califonia, USA. 1982, 2nd Ed. |
| [16] | H. Anthony (1996): Igneous petrology. Longman Group Ltd, England, 4th Ed. |
| [17] | I.G Ogezi (2004): Precambrian Geology of Nigeria- Geological Survey of Nigeria. Kaduna. pp 93-98 |
| [18] | M.A Rahman (2004): Geology of Nigeria. Elizabeth Publishing. Lagos. pp 45-50 |
| [19] | A. Carlos, C. Zuluaga, H.S Harold, Earth Science research Journal. 2001, 12(2):1-2 |
| [20] | S. Raymond (1974): Earth. Frank press, Cambridge, Massachusetts, 2nd Ed. |
| [21] | L.G Berry (2001) Concept, Description and Determinations. CBS Publishers, 4596/1-A New Delhi, India, 2nd Ed. |
| [22] | O.O Egunlae, O.A Oluwaseyi. Journal of Engineering and Earth Sciences. 2007, 2(1): 96-102. |


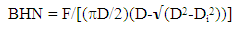
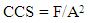
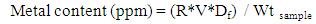
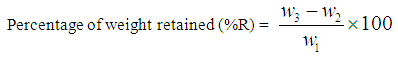







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



