-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Mining Engineering and Mineral Processing
p-ISSN: 2166-997X e-ISSN: 2166-9988
2014; 3(1): 1-5
doi:10.5923/j.mining.20140301.01
Study of Electrical Properties of Iron Ore in View of Electrostatic Separation
Idres A.1, Bounouala M.2, Boukelloul M. L.2, Talhi K.1
1Laboratory of Physical Metallurgy and Materials Properties (LM2PM) Badji Mokhtar University, BP 12, 23000, Annaba, Algeria
2Laboratory of Natural Resources and Development (LRNA) Badji Mokhtar University, BP 12, 23000, Annaba, Algeria
Correspondence to: Idres A., Laboratory of Physical Metallurgy and Materials Properties (LM2PM) Badji Mokhtar University, BP 12, 23000, Annaba, Algeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Electrostatic separation of materials is part of the non-conventional technologies used in the recycling industry; the current development is strongly stimulated by the regulations in the field of environmental protection. According to the climatic conditions of the region Ouenza (Algeria), a study was conducted by testing on samples, the possibility of treatment of these discharges electrostatic separation at high intensity. The results of measurement of the electrical properties obtained are very significant for electrostatic separation. Also by the encouraging results, the new process will enhance a share of volumes stored without the use of water and secondly to protect the environment from the dangers of these stocks.
Keywords: Iron ore, Mineral, Electrostatic separation, Semi-conductor, Conductivity environment
Cite this paper: Idres A., Bounouala M., Boukelloul M. L., Talhi K., Study of Electrical Properties of Iron Ore in View of Electrostatic Separation, International Journal of Mining Engineering and Mineral Processing , Vol. 3 No. 1, 2014, pp. 1-5. doi: 10.5923/j.mining.20140301.01.
Article Outline
1. Introduction
- The pithead of Ouenza currently stored in stocks currently several thousand tons of iron ore poor. These stocks have generated over the years, some negative effects on the environment of the region, these dumps can even be the main source of contamination of soil and surface water or groundwater or water remains a concern for most people and the industry.Natural minerals in their majorities are semiconductor materials whose characteristic is the high sensitivity electro physical properties to the presence of small amount of impurities and structural changes. The mineral crystals are individual objects so you can’t use a reliable baseline data on the electrical properties in the development of a technology that electrically separates a specific ore.The primary extraction of iron ore hub in Algeria is the deposit Ouenza. This field produces about 2 million tons per year of which 70% are higher grade than 55% and 30% variable content 45-50%. As for poor ore grade of 45% so they are temporarily stored on the floor of the mine.In this work, there is provided a study of the electrical properties of iron ore in view of electrostatic separation.Specific properties of iron ore have a great impact on the electrical aptitude: Mineralogical composition, character of distribution of minerals, useful content component, particle size distribution and the relationship between the content and the density of the field.
2. Experimental
2.1. Material
- The objective of this work is to study the electrical properties for electrostatic separation; we chose these materials for iron ore whose mineralogical composition (Table 1) was performed by means a metallographic microscope on polished sections. Chemical analysis by X-ray fluorescence shown in (Table 2) actually shows the poverty of ore and minerals especially iron hydroxides.Sampling was carried out according to known technique (taking on the job). From a representative sample, it was found that in a size range 100 - 120mm, it appears a possibility to extract common ground ore mineralogical varieties that differ considerably. That is why the research will be based on the sample of the given tranche.Manual sorting of a sample of ore dumps from Table 2 showed that we can extract four varieties: two metals and two non-metallic.The iron content in the original sample is 37.5 %, the basicity index 0.67 and 73.0 silicon module demonstrates this weak metallurgical properties and the need to upgrade the ore by ore- mechanical processes.Fraction hématito - hydrogoethite is represented as pieces of size 50 -100 mm and has relatively high iron content of 54%. The yield of this fraction in the sample is 8%. This fraction has a high alkalinity and 3.24 with mineral processing can obtain a concentrate of high metallurgical properties.
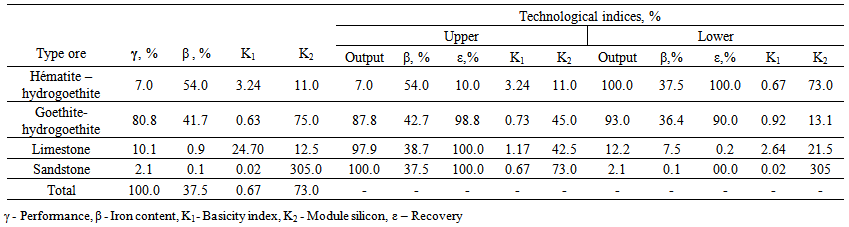 | Table 1. Indices of technological ore sample collected from the tailings deposit Ouenza [1] |
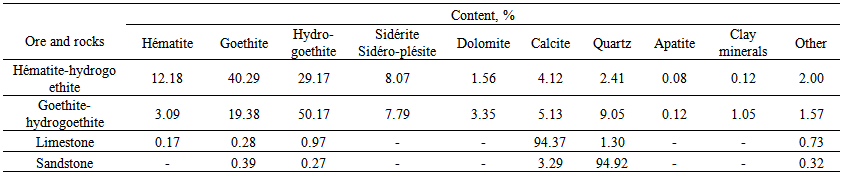 | Table 2. Mineralogical composition of ore and waste rock dumps of the deposit Ouenza [1] |
2.2. Electrical Properties of Minerals
- It is known that in the non- homogeneous body, and particularly in poly - crystalline minerals, it is possible not only superficial absorption, but also the diffusion of the gas components of the ambient atmosphere within the material. It is natural that in cases where the irregularities are distributed in a regular manner at least according to the volume of samples, the electrical conductivity is controlled by the state of its irregularities [2, 3].These factors may be determinants and hide when testing the manifestation of fundamental properties of the material volume. Moreover, solving the problem about the possibility of applying the method of electrical separation requires much the knowledge of fundamental features volume component commodity metal [4, 9].On the plane of the objective must be put know, first, the physical properties of raw materials of metal component in the external controlled conditions, and on the other hand, the qualitative variation of electrical conductivity with the external conditions change. These are especially important with regard to the choice of the system of separation.
2.3. Experimental Techniques and Methodology of Sample Preparation
- Flow of process in the physical separation of the ground material depends strongly on the electrical properties of the ore to be separated. Taking into account the fact that the metal materials are representative typical highly heterogeneous semiconductor with all their intrinsic properties at the development of an experimental technique, great attention was paid to the possibility of implementing an atmosphere controlled surrounding the sample.Experimental setup, whose diagram is given in Figure 1, was used to measure the electrical conductivity in a direct current (DC) at temperature ranging from minus 150 °C up to plus 180 °C.
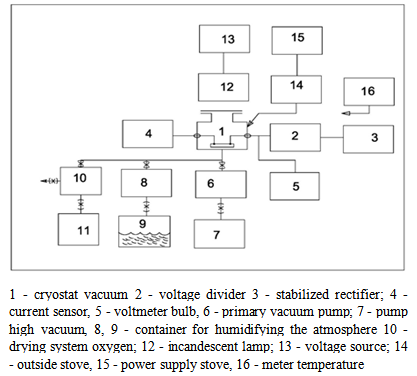 | Figure 1. Scheme of the experimental setup for studying the conductivity |
3. Results and Discussion
- The best results were obtained only in the first and the third case. In other cases (Reloading the target metals, low melting point and Application of the slurry on the basis of graphitic ammonium chloride) was proven instability high electrical contact. For samples with a dense structure, it was possible to use the method (Evaporation in vacuo of metals with the heated tungsten wire so that the Applying the conductive silver paste, while for the samples with a loose structure, vacuum evaporation does not continue with a conductive layer due the high concentration of micro pores on the surface. In this case we used the conductive paste (mixture of colloidal silver with an organic binder). Tests have shown that the contact of the silver conductive paste remains stable in the temperature ranging from 20 to 150°C.The planar geometry of the contacts are two conductive strips having a width of 2 to 3 mm applied to the working surface of the sample at a distance of 5 mm. The current collection contacts take place using the plates in the clamping spring bronze. To make measurements, the samples are installed on the frame of the cryostat vacuum.
3.1. Features Volt – Ampere
- In the semiconductor under heterogeneous irregular electric field distribution, the location of an electrical voltage in separate areas of the semiconductor can lead to effects of the powerful field of various kinds. The latter leads to false measurements of conductivity. To this end, for determining the area of the current flow ohmic regime, it is intended to measure the characteristics Volt - Ampere (CVA). The linearity of the CVA is the criterion of the absence of the effects of strong current [5, 6].The CVA is measured in a laboratory plant by slowly increasing the voltage and without interruption at room temperature. Generally, before measurement the sample is maintained at room temperature for 30-60 minutes in the dark, the light is also eliminated. Ambient parameters vary within: 56 - 57 kPa pressures, temperature 22 – 24°C; humidity 50-70 %. Figure 2 are shown the CVA samples under ambient conditions, it follows that they have a kind of power: I ≈ U n with a variable value n at low voltage and I ≈ 1 when a large voltage the value n is larger than a unit as is the case goethite (curve 2) and quartz (curve 4).
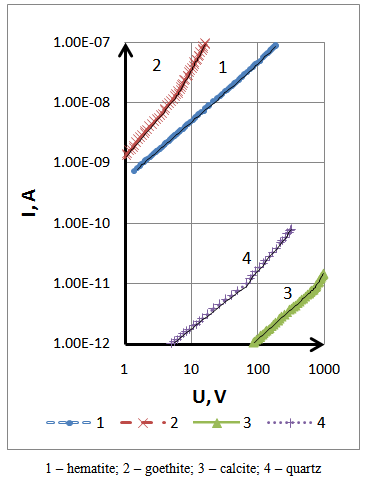 | Figure 2. CVA samples in ambient conditions |
3.2. Interdependence Conductivity - Temperature (ICT)
- The electrical conductivity depends greatly on the temperature 60-70°C. Therefore the search for optimal conditions of the technological process of separation depends on the study of relationships temperatures useful component in a wide temperature range [8].Testing dependencies - temperature conductivity (ITC) have conducted in the summers following conditions: temperature 24 – 180 °C at a constant speed from 0.2 to 0.3 degrees / second; Voltage 10 Volt; Exposure to room temperature 2 to 3 hours.Figure 3 shows the ICT in the atmosphere, curves 3 and 4 have a non-monotonous in nature. It is found that this is conditioned by the irregularity of the process of change in conductivity.
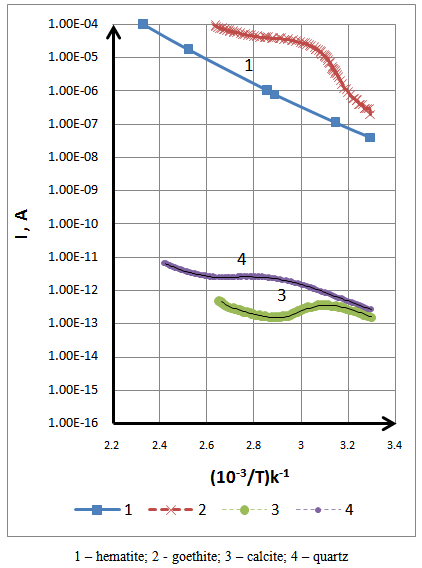 | Figure 3. Interdependence conductivity – temperature |
3.3. Changing the Conductivity of the Samples in the Humid Atmosphere
- The results of studies of the electrical conductivity achieved are obtained humidity 60-70 %. They indicate that the existence on the surface of the air absorbed molecules (oxygen, water), actually reduce the scale of the electrical conductivity of mineral studied.For the development of pretreatment process materials before separation, great importance is linked to variables, including the electrical conductivity change of thermal and atmospheric conditions [11, 12]. Therefore, the problem arises from how quickly diminishes the value of the conductivity after heat treatment.Conductivity is measured by increasing a gradual way the humidity of 60 - 100% of the time intervals. The measurement results are shown in Figure 4. The mineral components of low resistance (hematite, goethite) slightly change their electrical conductivity with time, while the high resistance component (calcite) increases the electrical conductivity due to the strong absorption of moisture. However, if the change in electrical conductivity for inorganic ferriferous happens insignificantly, in the case of high strength calcite occurs an abrupt increase in the electrical conductivity after 15 to 20 minutes.To protect the surface, the time of contacting with the atmosphere before the separation of mineral particles, it should not exceed several minutes. Under atmospheric conditions the ratio of electrical conductivity of iron-bearing components and the matrix is 4 times.
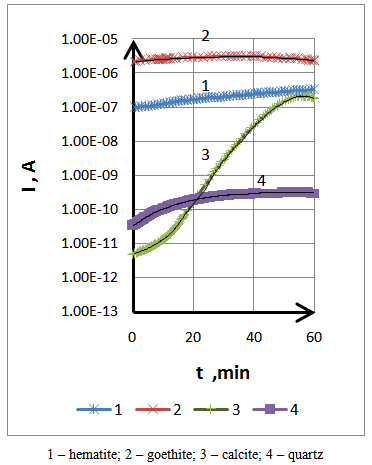 | Figure 4. Changing the conductivity of the samples in a humid atmosphere |
4. Conclusions
- 1. We developed the methodology and study of Vol- Ampere characteristics (CVA) and the temperature - conductivity ratio (RTC) of iron ore.2. It is established that the non- monotony of the RTC and the instability of CVA are conditioned by the mechanism of electron conductivity absorption of minerals.3. Was determined from the scale conductivity among ferriferous and gangue components which is two times more after the heat treatment. There is evidence that treatment of the inorganic filler by the corona contributes to the increase of the scale.4. It is established that the impact of moisture decreases abruptly graduation conductivity due to the increase of the conductivity of calcite. The effect of thermal treatment of the surface of the mineral before the electrical separation is kept at room temperature for 10-15 minutes.5. Any action causing the desorption of moisture from the surface of minerals studied can contribute to increasing the efficiency of the separation process.6. Experimental results have shown that it is necessary to ground the product to 0.25 mm and to consider the size fraction - 0.25 + 0.02 mm by performing the electric dust concentration before.
ACKNOWLEDGMENTS
- The work described in this article is part of the research program in the laboratory of Physical Metallurgy and properties of materials.It is our pleasure to express our gratitude to all those who in one way or another, have contributed to the completion of this work.We also wish to thank Distinguished Professors of the University of Mining Krivoy Rog - Ukraine for their interest in this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML