Md. Aminur Rahman1, Mohammad Nazim Zaman1, Md. Nehal Uddin2, Pradip Kumar Biswas1, Mst. Shanjida Sultana1
1Institute of Mining, Mineralogy and Metallurgy, Bangladesh Council of Scientific and Industrial Research, Khonjonpur, Joypurhat, 5900, Bangladesh
2Geological Survey of Bangladesh, 153, Pioneer Road, Segunbagicha, Dhaka, 1000, Bangladesh
Correspondence to: Md. Aminur Rahman, Institute of Mining, Mineralogy and Metallurgy, Bangladesh Council of Scientific and Industrial Research, Khonjonpur, Joypurhat, 5900, Bangladesh.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
In the present study, the main objective is the beneficiation of ilmenite from ultramafic lamprophyres of Mithapukur, Rangpur District of Bangladesh. Mineralogical studies performed by XRF, optical microscopy and Electron Probe Micro Analyzer (EPMA) studies indicated that ilmenite and apatite are main valuable minerals. The gangue minerals consist of the silicate minerals such as pyroxene, olivine and some secondary minerals. Ilmenite in ultramafic lamprophyre ore occurs in various forms such as Ilmenite grains, exsolved Ilmenite lamellae in phlogophyte and Ilmenite particles disseminated in silicate minerals. The feasibility of physical separation has been performed for mineral beneficiation. The grain forms liberated in 63µm-1000µm are recoverable by physical methods. The maximum content of TiO2 in Ilmenite lattice is determined 54.62% by EPMA. Although the ore has 6.37% average grade of TiO2, the recoverable TiO2 is only about 4.55%, the studied sample contained 11.10% Ilmenite and the amount of recoverable Ilmenite is only about 9.09% (4.55% TiO2). This is due to the Ilmenite exsolutions and inclusions in phlogophyte and silicate minerals, and the TiO2, in solid solution in the lattices of these minerals. In fact, about 81% of whole Ilmenite content of the ore will be recoverable.
Keywords:
Lamprophyre, Ilmenite, Apatite, Physical Separation
Cite this paper: Md. Aminur Rahman, Mohammad Nazim Zaman, Md. Nehal Uddin, Pradip Kumar Biswas, Mst. Shanjida Sultana, Beneficiation of Ilmenite from Ultramafic Lamprophyres of Mithapukur, Rangpur District of Bangladesh, International Journal of Mining Engineering and Mineral Processing , Vol. 2 No. 1, 2013, pp. 1-7. doi: 10.5923/j.mining.20130201.01.
1. Introduction
Titanium is widely used as titanium dioxide (TiO2) for production of white pigment[1]. Ilmenite (FeTiO3, 52.6% TiO2 and 47.4% FeO) is the most common source of titanium dioxide[2]. The term Ilmenite, as used in the titanium industry, commonly covers the entire range from unweathered Ilmenite with TiO2 contents below 50% to altered Ilmenite containing more than 60% TiO2[3]. All economically exploitable Ilmenite occur as hard rock resources and beach sands. The hard rock titanium deposits have complicated mineralogical characteristics whose identification is the most important from processing viewpoint. The amenability of various iron-titanium deposits to beneficiation is controlled by mineralogical and textural characteristics. In the evaluation of the mineralization and in the design of procedures of mineral dressing, the distribution of the valuable material is a matter of immediate importance. It is necessary to determine whether a given element is occurring in one mineral or several[3]. The Ultramafic lamprophyres hard rock deposits has been located at Mithapukur, Rangpur District of Bangladesh. The lamprophyres are alkaline potassic intrusives with affinities towards aillikites, lamproites and kimberlites and may be diamondiferous. Ultramafic lamprophyres of Mithapukur, Rangpur District of Bangladesh are extremely silica under saturated consists of olivine, phlogopite, pyroxene, biotite with apatite, rutile, leucite, spinel and some opaque minerals. The lamprophyres are essentially ultrapotassic (K2O/Na2O: 8-13) and ultramafic (MgO: 12.83-22.99wt %). High TiO2 (5.39-9.25wt %) as well as high P2O5 (1.99-3.15wt %) contents are key features of these rocks owing to high modal rutile + ilmenite and apatite respectively which is higher than beach sand or river sand. The identified ultramafic lamprophyre rock is discovered in the bore hole GDH-52 which was drilled by Geological Survey of Bangladesh (GSB). The higher contents of TiO2, P2O5 was found from the depth 722/ to 992/ of this borehole. The main objective of this study is to separate the TiO2, P2O5 bearing minerals from the Ultramafic lamprophyre hard rock. For this purpose the drill core sample has been collected from the core sample library of GSB. The objectives of the study are given below a. The quality and quantity of the liberated mineral and trace element of the rock.b. The estimation of the liberated mineral and trace element of the area.c. The up gradation of the liberated mineral into industrial grade. d. Whether it is economically viable or not, mineral beneficiation from the Ultramafic lamprophyre hard rock of Mithapukur.
2. Materials and Methods
The drill core samples from the borehole GDH-52 with overall length about 220/ (from the depth 722/ to 992/) were collected from core sample library of Geological Survey of Bangladesh, Bogra office. The crushing of the samples less than 2mm was done by laboratory jaw crusher. The disc mill was used for grinding of samples. The chemical and mineralogical composition of different samples carried out by X-ray fluorescence (XRF) and Electron probe micro analyzer (EPMA). The corresponding polished thin sections were studied for ore and rock forming minerals and their textural relationships by reflected and transmitted light microscopy.
2.1. Chemical Analysis by XRF
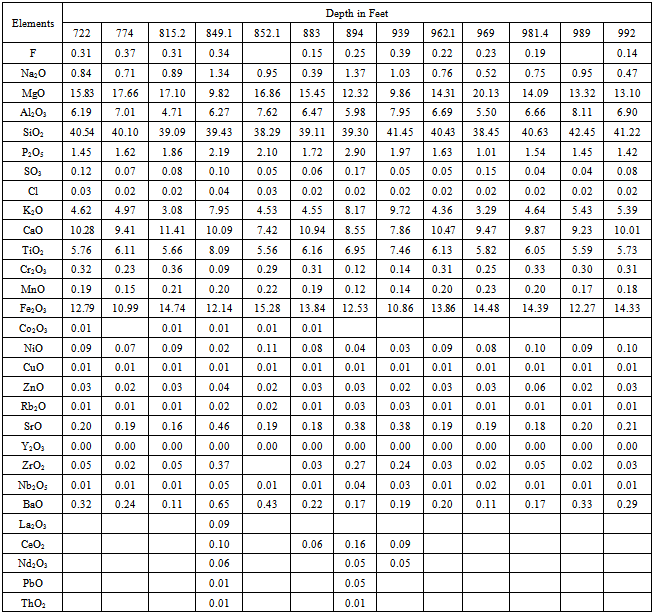
The collected drill core 13 (thirteen) samples depth from 722/ to 992/ were analyzed and the chemical composition of the samples is shown in Table 1. In the samples SiO2 ranges from 38.29 to 42.45%, Al2O3 4.71 to 8.11%, TiO2 5.56 to 8.09%, Fe2O3 10.86 to 15.28%, MgO 9.82 to 20.13%, CaO 7.41 to 11.41%, Na2O 0.39 to 1.37%, K2O 3.07 to 9.72% and P2O5 1.01% to 2.90% (Table-1). Table 1. showing the chemical analysis of the collected drill core samples
 |
| |
|
2.2. Ore Microscopy
Based on the results obtained from transmitted-light microscopy studies, these rocks have the mineralogy typical of highly-K plutonic ‘lamprophyre’ contains olivine, phologopite, pyroxenes, biotite, feldspar, leucite, apatite, spinel, rutile, chlorite and some opaque minerals. The dominant phenocryst is set in golden-yellowish in color of phlogopite, olivine and pyroxene (Fig. 4). Some of them with carbonate veins. Olivine, phlogopite and pyroxenes are the predominant phases with apatite and rutile. The phenocrysts are made up essentially of ferromagnesian minerals, an important characteristic of lamprophyres[10]. The porphyritic nature is highlighted by presence of hexagonal and pseudohexagonal clinopyroxenes and euhedral mafic minerals like olivine showing a panidiomorphic habit (Fig. 1).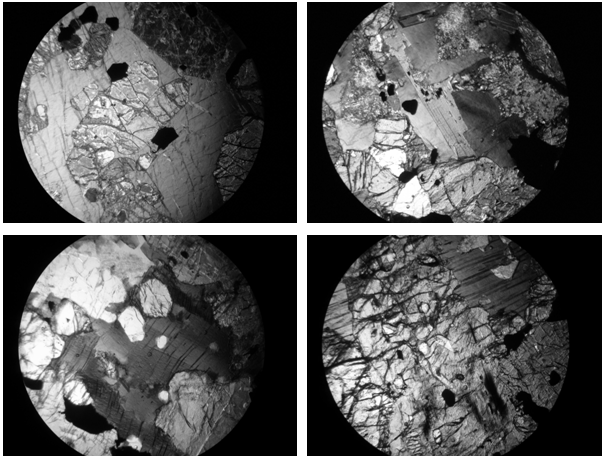 | Figure 1. Photomicrograph of lamprophyres a) euhedral grains of olivine and pyroxene (plane polarized × 4.0) b) panidiomorphic granular texture (plane polarized × 4.0) c) pyroxene-phlogopite-olivine nodule in lamprophyre consisting of large phlogopite plates with opaque oxides and apatite inclusions (cross nicols × 4.0) d) zircon inclusion in pyroxene (cross nicols × 4.0). Pyx-Pyroxene, Ol-Olivine, Chl-Chlorite, Bt-Biotite, Ap-Apatite, Opq-Opaque, Phl-Phlogopite,Zr-Zircon |
Subhedral and euhedral olivines (not rounded, as in kimberlite) and phlogopite occurs as phenocrysts (in some sections olivine grains are >1.5mm) as well as groundmass microphenocrysts of both. Quartz and amphibole/ hornblende grains are almost absent in the studied rocks. Phlogopite usually occurs as lath-shaped and orangish to golden yellowish in color with a tinge of red. The phlogopite grains are also observed in hand specimen with golden teen in color which is a characteristic feature of titanium-rich micas[11]. Both fresh and with euhedral apatite inclusions phlogopite grains are also observed in some thin sections. Some of the grains are twisted or bent probably due to some pressure. Some pyroxenes and phlogopite also alters to chlorite along the rim. The prismatic and euhedral apatite grains are abundant in all the samples of Mithapukur alkaline rocks; their abundance is also proved by high content of P2O5.
2.3. Electron Probe Microscopy Analysis (EPMA)
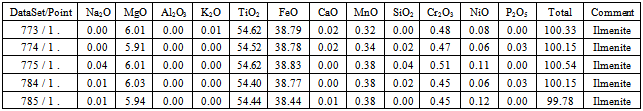
The studies of samples by EPMA were performed for two main purposes: 1) Study of exsolution and inclusion texture in ilmenite minerals, 2) to determine the minor element constituents in ilmenite and to distinguish the type of different lamellae inside them. Average analysis of different phases of samples performed by EPMA is given in Table 2. The TiO2 content of ilmenite is 54.44 to 54.62%. The MgO content of ilmenite is 5.91 to 6.03% which is relatively high.Table 2. showing the selected Electron Probe Microscopy Analysis (EPMA) of Ilmenite phase
 |
| |
|
3. Feasibility of Physical Separation
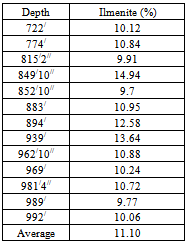
The crushing of the samples less than 2mm was done by laboratory jaw crusher. The disc mill was used for grinding of samples. The grinding samples were then oven dried over night at 110C and 100gm of each sample was sieved for 15 minutes on Fritsch Vibratory Sieve Shaker using mesh 45µm, 63µm, 500µm and 1000 µm sieving interval. The separation tests were performed on the head samples 500-1000µm size and 63-500 µm size fractions. The Ilmenite liberation degree was determined by using grain counting method by reflected microscope[7] (Table 3). The separation tests were performed by three methods such as hand panning method, gravitational separation by shaking table method and magnetic separation method.Table 3. showing Ilmenite percentage of the collected drill core samples
 |
| |
|
3.1. Hand Panning Method
The hand panning test was performed for 9 (nine) samples to separate the heavy and light part. The average grading TiO2 of the nine samples is 6.37% which implies about 11.10% Ilmenite (Table 3). The two size fractions (500µm-1000µm and 63µm-500µm) have been taken for this test. The result shows that in the size fraction 500µm-1000µm, the concentration of heavy part is on an average 60.04% and in the size fraction 63µm-500µm is 20.75% (Table 4). The chemical analysis of heavy part was done by XRF. The heavy part contains 9.98% of TiO2 (Table 5). The recovery of TiO2 in heavy part of the size fraction 500µm-1000µm is 68.46% and in the size fraction 63µm-500µm is 27.45%. So in the hand panning method, the recovery of TiO2 in the size fraction 500µm-1000µm is better than the size fraction 63µm-500µm. In this experiment, though the TiO2 can be recovered in the heavy part but the bulk amount of the concentration is high. As a result, the extraction of TiO2 from heavy part will be more costly.Table 4. showing the heavy and light part separation by hand panning method
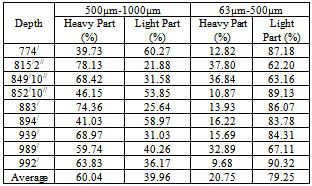 |
| |
|
Table 5. showing the chemical analysis of heavy and light part separated by hand panning method
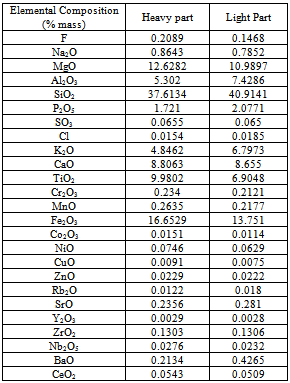 |
| |
|
3.2. Gravitational separation by Shaking Table
The gravitational separation test by shaking table was performed for 3 (three) samples to separate the heavy and light part. The average grading of TiO2 of the 3 (three) samples is 5.90%.The result shows that the concentration of heavy part of three samples is 22.70 to 24.46% (Table 6). Table 6. showing the heavy and light part separation by shaking table
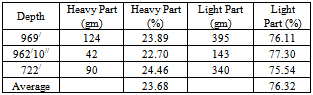 |
| |
|
After separation the concentration of heavy part of the three samples is mixed and the chemical analysis of heavy part was performed by XRF. The TiO2 contain in the mixed heavy part is 16.26% (Table 7). The recovery of TiO2 in the heavy part of mixed sample is 51.46%. Compare to hand panning method, this method is better to extract TiO2 economically but this concentrate should be run at magnetic separator. Table 7. showing the chemical analysis of heavy and light part separated by shaking table
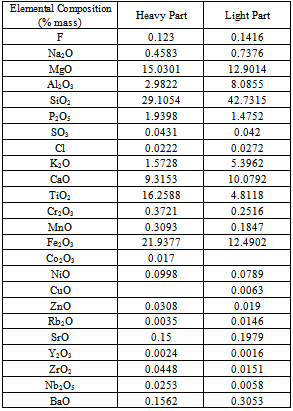 |
| |
|
3.3. Magnetic separation by Induced Roll Magnetic Separator (IRMS)
The magnetic separation test by induced roll magnetic separator of the samples was done to separate the magnetic and non-magnetic part. The two size fractions (500µm-1000µm and 63µm-500µm) of 9(nine) samples have been taken for this test and run at 3 amps current to the induced roll magnetic separator. The recovery of magnetic fraction is well in the size fraction 63µm-500µm (average 61.38%) than the size fraction 500µm-1000µm (average 26.28%) (Table8). Table 8. showing the magnetic separation of the drill core samples by IRMS
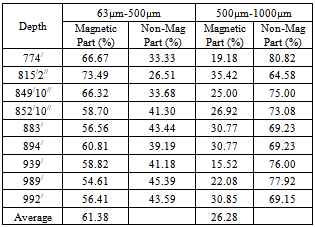 |
| |
|
As the recovery of magnetic fraction in the size fraction 63µm-500µm is high, this fraction was taken for further magnetic separation. The sieve analysis was done for this fraction of 8(eight) samples. After sieving, the three size fractions 63µm-125µm, 125µm-250µm and 250µm-500µm were obtained from eight samples (Table 9). Table 9. showing the grain size analysis of magnetic fraction of eight samples
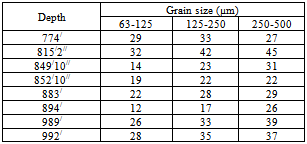 |
| |
|
Then individual fraction of each samples were mixed to get the head samples of that three size fractions. The samples were run at 0.3 amp current to the induced roll magnetic separator (Table 10) and did XRF analysis of the separated magnetic part (Table 11). Table 10. showing the magnetic separation at 0.3 amp by IRMS of three size fractions
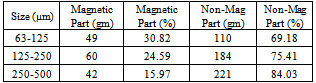 |
| |
|
The result shows that in the size fraction 63µm-125µm, the product is an Ilmenite concentrate with 15.47% TiO2 grade by containing 78.45% (Recovery) of titanium dioxide. In the size fraction 125µm-250µm, the product is an Ilmenite concentrate with 21.81% TiO2 grade by containing 78.93% (Recovery) of titanium dioxide. In the size fraction 250µm-500µm, the product is an Ilmenite concentrate with 28.53% TiO2 grade by containing 81.87% (Recovery) of titanium dioxide. The beneficiation of Ilmenite in this method is higher than other methods but the recovery is depending on the grain size fractions. It is difficult to keep the grain size in definite size fractions by disc mill or any other grinding mill in commercial operation.Table 11. showing the elemental composition of the separated magnetic part of three size fractions
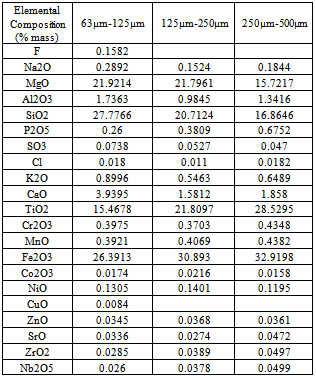 |
| |
|
4. Conclusions
According to ore microscopically results, these rocks have the mineralogy typical of highly-K plutonic ‘lamprophyre’ contains olivine, phologopite, pyroxenes, biotite, feldspar, leucite, apatite, spinel, rutile, chlorite, ilmenite and some opaque minerals. The dominant phenocryst is set in golden-yellowish in color of phlogopite, olivine and pyroxene. The ultramafic lamprophyre ore by average grading of 6.37% TiO2 which implies about 11.10% Ilmenite (by considering 48% TiO2 for Ilmenite, based on EPMA analysis result) is one of the low grade deposits in the world. However the TiO2 content of Lamprophyre of Mithapukur deposit is lower than Tellnes (upto 18% TiO2 and 35% Ilmenite) and Otanmaki (upto 14% TiO2 and 28% Ilmenite) ores but it is comparable with these ores from reserve viewpoint.The Ilmenite in this ore is in various forms: Ilmenite grains, exsolved Ilmenite lamellae in phlogophyte, Ilmenite fine particles disseminated in silicates. Based on TiO2 content of Ilmenite concentrate, the amount of lamellae form of Ilmenite is unrecoverable by physical methods. The much of the Ilmenite disseminated in silicate minerals are unrecoverable by physical methods too. So, the TiO2 content of the ore which is usually determined by chemical analysis is not totally recoverable. Consequently, the recoverable Ilmenite content of the rock is not exceeding 9.09%. Another valuable mineral apatite can not be recovered by physical method. In spite of complicated mineralogical features of studied ore, it is predicted that by combination of gravity methods such as tabling and Humphrey spiral and magnetic separation, the concentration and production of commercial Ilmenite concentrate from ultramafic lamprophyre deposit will be possible. In addition, the P2O5 is obtained from apatite concentrate which can be recovered by floatation of tailings of gravity separation. Ilmenite and apatite are valuable minerals for production of TiO2, P2O5 and Fe; the rare earth elements Sr, Rb, Nb, Nd, Ce, Ba can be extracted as a by-product. The ultramafic lamprophyre which is discovered by IMMM scientists is alkaline potassic intrusives with affinities towards aillikites, lamproites and kimberlites and may be diamondiferous. Throughout the world diamond is explored and mined in this type of rock. So this area may be a prospective zone of Bangladesh for valuable minerals and metals. This study is based on the one drill hole done by Geological Survey of Bangladesh, that’s why the reserve estimation and extent can not be possible. For this purpose a comprehensive study such as drilling program in systematic pattern and geophysical exploration are needed to estimate the reserve calculation.
References
| [1] | Colin J Douch, 2001; “Ilmenite, Titanium Dioxide and Titanium”; New Zealand Mining, Vol.30; pp.30-37 |
| [2] | Chernet Tegist, 1999; “Applied Mineralogical Studies on Australian Sand Ilmenite Concentrate with special reference to Its Behavior in the Sulphate Process”; Minerals Engineering, Vol.12, No.5; pp. 485-495 |
| [3] | Chernet Tegist, 1994; “Ore Microscopic investigation of selected Fe-Ti Oxides bearing samples from Konusaarenneva Mineralization and other Localities in south western Finland”; Geological Survey of Finland, Department of Mineral Resources, Section for Industrial Minerals. |
| [4] | Zaman, M,N., Miah, M.Y., Ahmed, S.S., Uddin, M.N., Alam, A.K.M.B. and Biswas, P.K., 2010. “Mineralogy and geochemistry of the sub-surface Lamprophyres of Mithapukur, Rangpur District, Bangladesh”. Geoscience Journal, Jahangir Nagar University, Savar, Dhaka, Bangladesh |
| [5] | Goto, A. and Tatsumi, Y. (1994). Quantitative analysis of rock samples by an X-ray fluorescence spectrometer (I). The Rigaku Journal 11, 40-59. |
| [6] | Goto, A. and Tatsumi, Y. (1996). Quantitative analysis of rock samples by an X-ray fluorescence spectrometer (II). The Rigaku Journal 13(2), 20-39. |
| [7] | Mange, A. M. and Maurer, H. E. W. (1991) Schwerminerale in Farbe: Stuttgart (Ferdinand Enke Verlag). |
| [8] | Mineral Facts and Problems (1980) Edition, Bureau of Mines, United States Department of Interior. |
| [9] | Mineral Sands in Asia and the Pacific, United Nations, Economic and Social Commission or Asia and the Pacific, ST/ESCAP/548, p. 103-108. |
| [10] | ROCK, N.M.S., 1977, The Nature and Origin of Lamprophyres: Some definitions, distinctions and derivations; Earth-Science Reviews, 13, 123-169. |
| [11] | MITCHELL, R.H. & BERGMAN, S.C., 1991, Petrology of lamproites; New York, 447p. |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML










