-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Mining Engineering and Mineral Processing
p-ISSN: 2166-997X e-ISSN: 2166-9988
2012; 1(3): 107-114
doi: 10.5923/j.mining.20120103.02
Baseline Studies of Some Heavy Metals in Top Soils around the Iron - ore Mining Field Itakpe North Central Nigeria
Olatunji Olatunde Stephen 1, Osibanjo Oladele 2
1Department of Chemistry Faculty of Applied Sciences Cape Peninsula University of Technology Cape Town, Western Cape, South Africa
2Department of Chemistry Faculty of Science University of Ibadan, Ibadan Oyo State Nigeria
Correspondence to: Olatunji Olatunde Stephen , Department of Chemistry Faculty of Applied Sciences Cape Peninsula University of Technology Cape Town, Western Cape, South Africa.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The baseline concentration levels of cadmium(Cd), manganese(Mn), chromium(Cr), nickel(Ni), copper(Cu) zinc(Zn) and lead(Pb) were determined in the top soil around the iron ore deposit at Itakpe North Central Nigeria in order to define benchmark concentrations as a basis for future environmental monitoring and pollution control for sustainable environmental protection. One hundred and sixty soil samples were randomly collected over an area of 5x5km2 between December 2003 and November 2005. The soil samples were subjected to standard methods and analysed using Unicam 969 flame atomic absorption spectrophotometer. The pH and organic carbon concentration of the soil ranged 4.28-7.46 (6.56±0.70) and 2.60-5.60% (2.85±1.00%) respectively. The mean concentration levels of heavy metals ranged: Zn, 43.89±9.06-75.29±15.74 mg/kg; Cu, 33.48±7.44-51.50±7.35 mg/kg; Pb, 18.73±2.87-33.31±4.31 mg/kg; Mn, 6.39±1.07-20.31±3.42 mg/kg; Cr, 8.18–14.89 mg/kg; Ni, 11.99±2.71-20.84±2.09 mg/kg; and Cd, 0.10±0.05-0.21±0.07 mg/kg. The soil metals concentration sequence was Zn > Mn > Pb > Ni > Cu > Cr > Cd, with Zn having the highest relative abundance in topsoil in respect to the measured metals while Cd had the least. There was decreasing gradient in the heavy metals concentrations from top soil (0–15cm) to depth 110cm. Zinc concentration level decreased by 23.92% from 67.65 mg/kg top soil to 13.06 mg/kg at 100–110 cm; Mn, 21.5% from 20.30-4.30 mg/kg; Cr, 14.09% from 14.89-1.84 mg/kg; Ni, 16.65% from 16.88-2.41 mg/kg; and Cu, 19.54% from 45.99-7.52 mg/kg, from top soils to depth of 100–110 cm respectively. Cadmium and lead were found below the instruments detection limit (i.e. < 0.002, Cd and < 0.05, Pb) at depth 50–110 cm and 100-110 cm respectively. Apparently, the soil environments are yet to be impacted negatively by heavy metals because heavy metal levels around Itakpe iron ore deposit and beneficiation plant were within natural concentration levels, and are therefore regarded as not polluted.
Keywords: Baseline, Concentration Levels, Top Soil, Benchmark, Polluted
Cite this paper: Olatunji Olatunde Stephen , Osibanjo Oladele , "Baseline Studies of Some Heavy Metals in Top Soils around the Iron - ore Mining Field Itakpe North Central Nigeria", International Journal of Mining Engineering and Mineral Processing , Vol. 1 No. 3, 2012, pp. 107-114. doi: 10.5923/j.mining.20120103.02.
Article Outline
1. Introduction
- The recognition of the strategic importance of the iron and steel industry in the diversification of Nigeria’s hydrocarbons-dominated economy informed the decision of the Federal Government of Nigeria to establish an iron and steel industry in Nigeria. The mandates include the detailed exploration for iron ore and steel raw materials, iron-ore beneficiation and processing to produce steel. The success of the detailed exploration for iron culminated in the discovery of the Itakpe hills iron ore deposit, and many other iron ore deposits in the same geological environment of North Central Nigeria. Two main varieties: oolitic ironore and ferruginous quartzite were identified. The Itakpe hills iron ore deposit is of a workable ferruginous quartzite type. The location of the iron ore deposits geological veins influenced the siting of the iron mining company at Itakpe. However, the commercial mining of iron-ore for steel production at Itakpe[1 - 2] had been severely restrained by lack of funding and the non-completion of associated infrastructure[3]. The extraction of many important metals has increased exponentially since the 1700s; with anthropogenic releases in many cases outstripping natural flow. Nriagu[4] reported that the direct activities of extraction, processing for industrial and consumer use contributes to the global mobilization of heavy metals. Thus, the rapid modern rise in industrial mining capacity has meant geometric expansion in both numbers and quantity of metals extracted from the earth and put to use. Industrial scale mining activity is comparatively low in Nigeria, yet at this level of mining, the nation is increasingly becoming exposed to the unwanted ecological effects of heavy metals. Studies revealed that heavy metals such as cadmium, lead, nickel, manganese, copper, zinc, tin, chromium, and arsenic are natural components of iron ore deposits, and are thus released into the environment in the process of mining/extraction, smelting and refining of ore. Kabata-Pendias and Pendias[5] reported the association of Ti2+, V3+, Cr3+, Mn2+, Co2+, Pb2+, W5+ and U4+ with iron (II) (Fe2+), and Ti4+, V4+, Cr3+, Mn2+, Co2+, Pb2+, W5+ and U5+ with iron (III) (Fe3+) cations. Thus, the potential for contamination of the environment is increased, because iron ore mining exposes metal-bearing ores[6]. Leaching of mine tailing and drainage from mined areas can also introduce substantial amount of metals into the immediate environment i.e. land, air and water[7]. On the other hand, nature’s release of metals by the process of rock weathering, soil erosion or the dissolution of water soluble salts[7, 8] is limited, because natural process of weathering of crustal materials is slow and occurs over time. Arhens[9] reported that natural release into the environment is in proportion of metal concentrations in soil forming rocks. Consequently, metals are also released into the environment from anthropogenic sources at levels several fold higher than nature’s input. The concerns for heavy metals arise from their persistence and harmful effects on the environment and human health[10, 11]. Trace amounts of heavy metals can accumulate in the food chain[12, 13, 14], eventually causing diseases such as autism, cancer, dementia, dyslexia, leukaemia, lymphoma etc and health condition such as neuro-degeneration and senility[15, 16]. Metals such as selenium, zinc, manganese, copper, nickel etc are essential for proper metabolism and development in living organisms[17, 18, 19], but are toxic and deleterious at elevated concentrations. According to Biney, et al.[20] and Edorh[21], many African countries including Nigeria are yet to conduct rational systematic studies to determine the extent and effects of metal pollution, neither have they conducted any assessments of the potential pollutants that characterize their ecosystems. Consequently environmental and health data on levels, characterization and the threats created by heavy metals are rare and scattered in Nigeria and Africa as a continent. There is need to identify the sources and the quantity of heavy metals dumped in the continent’s terrestrial and aquatic ecosystems, and to take measures to prevent this pollution.
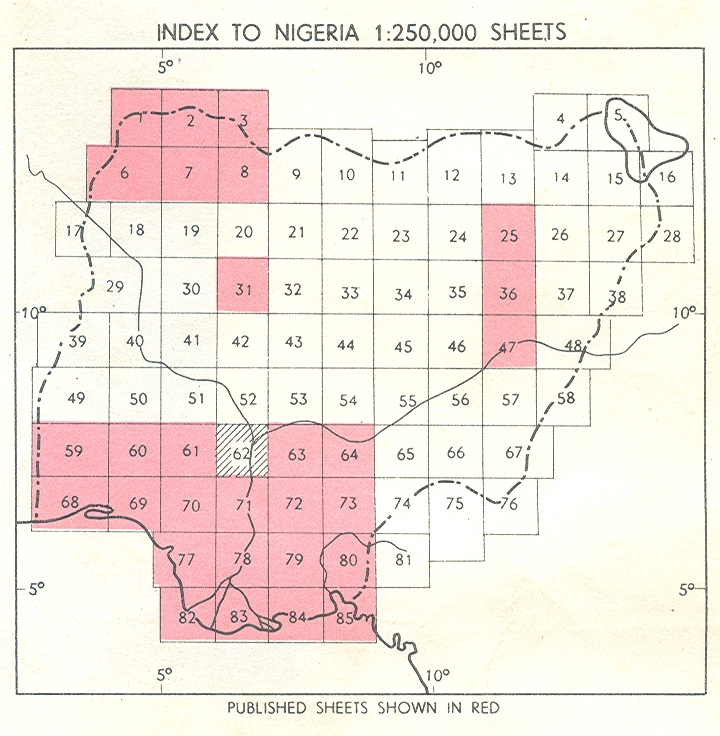 | Figure 1. Map of Nigeria (Inset: Location of Study area: 62): Source GSN, 1986 |
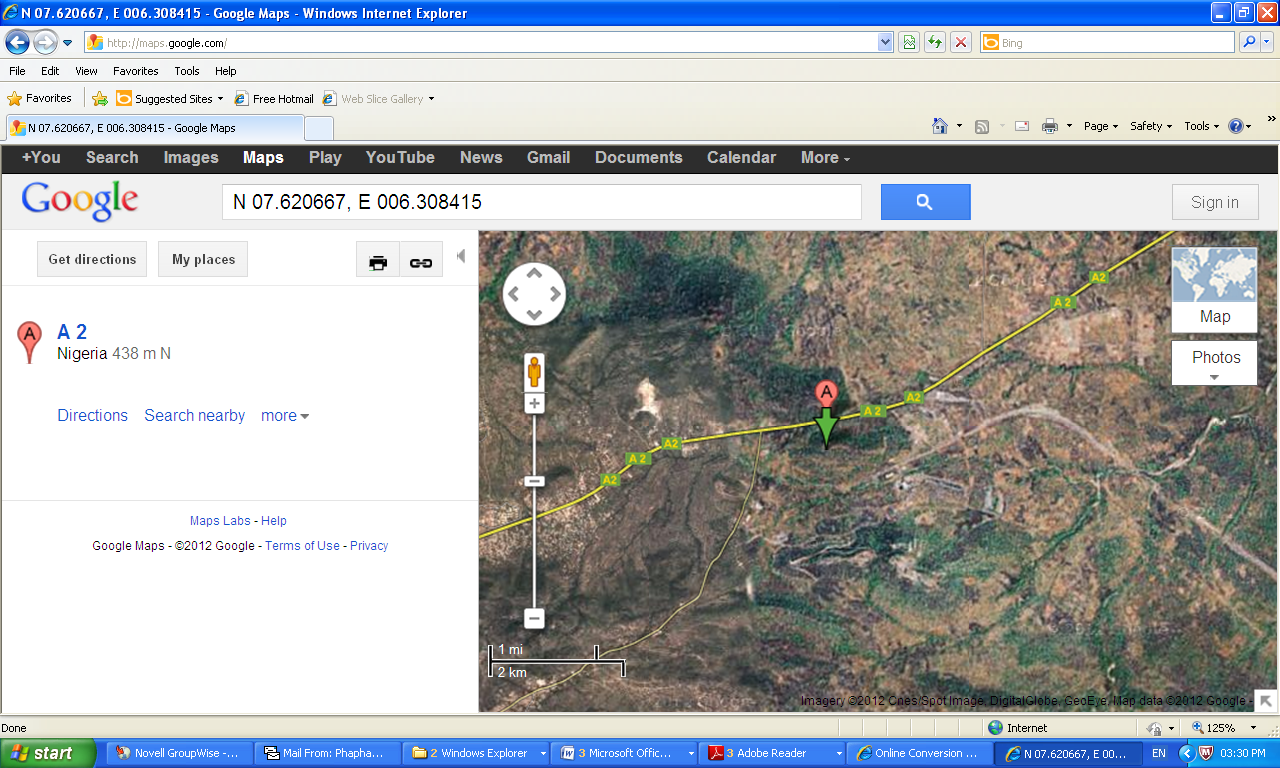 | Figure 2. Location map of the sampling area at Itakpe and Ajaokuta in Kogi State, North Central Nigeria |
2. Materials and Methods
2.1. Study Area
- The study area which covers Itakpe Iron-ore mining deposit and the industrial are of the Nigeria Iron-ore mining Company is located within the Lokoja–Okene Geological Survey Area in Nigeria (Figure 1) delimited by latitudes 70 and 80N and longitudes 60 and 70E. The area is made up of crystalline basement complex with compositional variability and structural complexity covering over 7,770 square kilometers[22] and sedimentary sequence consisting of sand stones and mudrocks of Cretaceous to tertiary age covering about one third of the area[23].
2.2. Sampling Location
- Sampling was centred around Itakpe iron ore deposit and the industrial area of the Nigerian Iron ore Mining Company located on N 07.6236670, E 006.3047330 to N 07.6204520, E 006.3085170 and altitude ranging from 241-325 m of Itakpe iron ore deposit/mining field, North Central Nigeria, Figure 2.
2.3. Samples Collection
- Top soils from 0–15 cm of A–horizon were randomly collected by scooping surface soils of the sampling areas using a stainless steel hand trowel. The soil samples were stored in a nitric acid pretreated and dry polypropylene bags and well labeled. A total of one hundred and sixty soil samples were collected over the 24 months sampling period between December 2003 and November 2005 encompassing the two climatic seasons of the year i.e. dry and wet seasons.For subsurface evaluation of heavy metal availability and migration at different substrata depth, surface (0-15 cm) and subsurface samples at depth 50-55 cm and 95-100 cm were collected at Itakpe about N 070 33”, and E 0060 41”. The samples were stored in separate nitric acid pre-treated and dry polypropylene bags and well labeled.
2.3.1. Primary Sample Preparation
- Soil samples[24, 25] were manually sorted to eliminate pebbles and coarsy materials, and air-dried under ambient conditions that are inside the laboratory for seventy-two hours. The dried soil and sediment samples were pulverized in a disc mill crusher. The resulting powdered samples were screened through a nylon sieve of 2 mm mesh size. 5 g each of soil sample was weighed into 250 mL teflons beakers earlier treated by soaking in dilute nitric acid (0.001 M) overnight and dried in oven at 500C. 50 mL 2 M nitric acid (British Drug House BDH) analar grade reagent was added to each beaker and heated in boiling water in a water bath for two hours[24, 25, 26]. The resulting sample digests were filtered into 100 mL volumetric flasks and made up to 100 mL mark with distilled water. Procedural blank was prepared by heating 15 mL 2M nitric acid in 50 cm3 distilled water for two hours, filtered and made up to 100 mL mark with distilled water.
2.3.1.1. Instrumental Analysis
- The digested sample solutions of soil in 100 mL volumetric flasks were quantified for the heavy metals cadmium Cd, chromium Cr, manganese Mn, nickel Ni, copper Cu, zinc Zn and lead Pb, by use of flame atomic absorption spectrometry (FAAS) by aspirating the samples into flame of Unicam 969 atomic absorption spectrometer fitted with deuterium lamp, over a 2mm burner using pre-mix fuel (i.e. air/acetylene mixture).
2.3.2. Determination of Soil pH and Organic Carbon
- Hydrogen ion concentration was determined using the method of McLean[27]. 1:1 Mixture of soil and distilled water and sediment and distilled water was prepared by weighing 20 g of fine grained (pulverized) soil/sediments into a glass beaker and 20 mL of de-ionised distilled water was added to each and stirred gently to enhance H+ (Hydrogen ions) release from soil. Soil pHs were measured after the resulting mixtures were allowed to stand for 30 min using a pH meter.
2.3.3. Determination of Organic Carbon in Soil
- Soil organic carbon was determined using the method of Walkley-Black[28]. 5 g each of soil samples were weighed into 250 mL teflon beakers. Each of the sample beakers were subjected to rapid dichromate oxidation by addition of 50 mL potassium dichromate 0.5 M K2Cr2O7 (May & Baker) and 2.5 mL concentrated sulphuric acid in 5% FeSO4 (BDH and May & Baker). The resulting solution was swirled and allowed to stand for a while to reduce the heat generated by exothermic reaction. Gentle boiling of samples for 30 min at 150℃ followed this and water added to the digestive mix to halt the reaction. Phosphoric acid (H3PO4) (BDH chemicals) was added to each of the digestive mix when cooled to eliminate interference from iron III (Fe3+) ions that may be present. Excess Cr2O72- was titrated with 0.25 M Ferrous ammonium sulphate using barium diphenylamine sulphonate as indicator.
|
2.4. Recovery Studies
- Recovery studies conducted on spiked soil samples validated the efficiency of sample the digestion procedures. The coefficient of variation and recoveries of the replicate analyzed samples ranged between 0.70% - 17.54% and 90.25 - 109.83% respectively. The recovery of each metal was within the acceptable recovery of 100±20%.
3. Results and Discussion
3.1. Results
- The results of analysis of Itakpe soils (sandy loam, sandy clay loam and clay loam) are presented in Table 1. The mean concentrations (mg/kg) of heavy metals (range in parenthesis) detected in the soils during dry season 2003/2004 were: Pb, 24.45±1.66 (22.49-28.51); Cu, 51.50±7.35 (37.94-61.05); Ni, 20.84±2.09 (16.91-26.23); Zn, 75.29±15.74 (64.17-92.68); Cr, 11.54±1.21 (9.71-14.08); Mn, 11.37±1.36 (6.07-9.12); and Cd, 0.16±0.05 (0.09-0.26): while during dry season 2004/2005; Pb, 33.31±4.31 (30.82-41.97); Cu, 45.99±10.08 (31.73-67.16); Ni, 16.88±3.54 (11.12-23.24); Zn, 67.65±13.59 (48.74-86.20); Cr, 14.89±1.66 (12.59-17.57); Mn, 20.31±3.42 (11.67-25.49); and Cd, 0.21±0.07 (0.11-0.32). The mean concentrations (mg/kg) of heavy metals (range in parenthesis) measured in the soil samples during wet season 2003/2004 were: Pb, 18.73±2.87 (13.77-25.03); Cu, 33.48±7.44 (17.57-45.03); Ni, 15.42±2.11 (11.68-19.85); Cr, 8.17±1.59 (4.18-10.54); Mn, 6.39±1.07 (4.78-8.24); Zn,43.89±9.06 (34.52-53.04); and Cd, 0.10±0.05 (0.03-0.21); while heavy metals levels during wet season 2004/2005 were: Pb, 23.98±3.56 (18.80-31.76); Cu, 37.95±7.60 (21.08-49.57); Ni, 11.99±2.71(8.10-18.06); Zn, 54.56±11.05 (38.95-70.24); Cr, 10.49±1.65 (6.17-12.68); Mn, 13.26±1.83 (10.40-16.98); and Cd, 0.16±0.07 (0.05-0.29).
3.2. Discussion
- The concentration levels of Zn was highest in respect of the metals measured, with mean concentration ranged 43.89 ± 9.06 - 75.29 ± 15.74 mg/kg, followed by Cu which ranged between 33.48 ± 7.44 - 51.50 ± 7.35 mg/kg, and Pb, 18.73 ± 2.87 - 33.31 ± 4.31 mg/kg. The mean concentrations of other metals ranged: Mn, 6.39 ± 1.07 - 20.31 ± 3.42 mg/kg; Cr, 8.18–14.89 mg/kg; Ni, 11.99 ± 2.71 - 20.84 ± 2.09 mg/kg; and Cd, 0.10 ± 0.05 - 0.21 ± 0.07 mg/kg being the least (Table 1). The concentration distribution sequence trend were Zn > Mn > Pb > Ni > Cu > Cr > Cd, and is consistent with the findings of Ma and Rao[29] in the order Zn > Cu > Cd > Ni, with Zn having the highest respect to the relative abundance in topsoil. This showed that the measured metals are heterogeneously distributed in soils around the iron ore-mining field and the ore processing industrial area. According to Paulo et al.[30], variability in quantity of heavy metals concentration on soil surface is largely determined by the physicochemical properties of the each heavy metal such as volatility, solubility, electro-positivity, ionic radii, redox potential and bond energy between heavy metals and soil. Soil properties such as soil pH, relative amount of soil organic carbon, soil particles size, clay mineral, clay mineral composition also partly account for the concentrations of heavy metals in soils[20]. Thus, in soils where many metal cations are available, selective retention of metals may be competitive, and this may determine their distribution in soils, availability to plants as well as their movement throughout the soil. Soil within the study area is mildly acidic. The pH of the sampled soils (mean in parenthesis) ranged 4.28-7.46 (6.56±0.70), while the soils composition level of organic carbon (Tables 1) ranged 2.60 - 5.60% (2.85±1.00%). Fuller et al.[31], Huete and Mccoll[32] and He et al.[33], reported that pH has significant influence on metals solubility and soil anion exchange capacity and thus the relative abundance of heavy metals in soils. The sorption capacity of soils is a function of soil organics and clay minerals, and this defines retention capacity of soils. Chiou and Kile[34] reported that the sorption of heavy metals to soil as a result of organic carbon content is probably one of the factors that determine heavy metals concentration levels in soil environment, although this depends on the prevailing soil pH. Sorption to soil affects not only the contaminant level in an ecosystem, but the movement and fate of the contaminant as well. There was significant correlation (p < 0.05) between the concentration levels of heavy metals in soils and organic carbon levels, with correlation coefficient for Cd, γ = 0.74, Mn, γ = 0.51, Cr, γ = 0.70, Ni, γ = 0.69 and Cu γ = 0.56. Apparently, the concentration levels of the heavy metals in the soil samples were partly a function of the soil acidity and organic carbon levels.
|
4. Conclusions and Recommendation
- The concentration levels of Cd, Mn, Cr, Ni, Cu, Zn and Pb in soil around Itakpe iron-ore deposit and industrial area were low and within natural concentration levels. The soils are not considered as contaminated, thus the soil environment around Itakpe iron ore deposit and mining field are yet to be impacted negatively by heavy metals. This study therefore report the baseline concentration levels of Cd, Mn, Cr, Ni, Cu, Zn and Pb in soils around the iron ore deposit and mining field at Itakpe, North Central Nigeria, which was hitherto non-existent. The baseline concentration data will serve as monitoring benchmark during mining and ore processing operations. There is a need to develop an environmental monitoring and management programme for heavy metals. This is in order to monitor the on-set of heavy metal pollution that could endanger the immediate environment, and as well sustain the status of the environment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML