-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Mining Engineering and Mineral Processing
p-ISSN: 2166-997X e-ISSN: 2166-9988
2012; 1(1): 17-20
doi: 10.5923/j.mining.20120101.02
Role of Water Structure-Making/Breaking Ions in the Cationic Flotation of Kaolinite: Implications for Iron Ore Processing
Mark Ma , Warren J. Bruckard, David McCall
CSIRO Process Science and Engineering, Box 312, Clayton, Victoria, 3168, Australia
Correspondence to: Mark Ma , CSIRO Process Science and Engineering, Box 312, Clayton, Victoria, 3168, Australia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Australia is the largest exporter of iron ore in the world. The high Al content in Australian hematite/goethite ores is detrimental to blast furnace and sinter plant operations. Of the Al containing minerals in iron ore, kaolinite is a common gangue mineral frequently found in iron ore deposits. In iron ore flotation pulp, various ions exist and may interfere with the flotation performance when their concentration is high enough. In this work, the role of a water structure-making ion, i.e. Na+, and a water structure-breaking ion, i.e. K+, in the flotation of kaolinite under reverse cationic flotation conditions, the most widely used flotation route of iron ore in the world, was studied in a series of laboratory batch flotation tests. It was found that K+, a water structure breaker, can better reduce the zeta potential of kaolinite and thus causes higher flotation recovery of the clay mineral, in comparison to Na+, a water structure maker. The different effects of the alkali metal cations on kaolinite flotation are attributed to the different aggregation degree of kaolinite particles in the presence of these cations.
Keywords: Kaolinite, Flotation, Amine, Water Structure Maker, Water Structure Breaker
Article Outline
1. Introduction
- Australia is the largest exporter of iron ore in the world. A significant problem with the Australian hematite/goethite ores is their high Al content. The high Al content in iron ore results in a highly viscous slag and high coke rate and thus is detrimental to blast furnace and sinter plant operations. Of the Al containing minerals in iron ore, kaolinite is a common gangue mineral frequently found in iron ore deposits [1-9]. The water structure-breaking or structure-making capabilities of the ions in iron ore flotation pulp are usually ignored when discussing the performance of iron ore flotation. The concept of “water structure breakers” and “water structure makers” was first introduced by Gurney, and Frank and Wen who postulated that each ion is surrounded by three distinct regions of water structure[10,11]. In the first layer, water molecules are tightly bound to the ion. The second region extends farther away from the ion and is referred to as the region of structure breaking. Only at larger distances, where the ionic field is weak, water molecules form the “normal” ice-like structure. Small (in terms of crystallographic radii), strongly hydrated ions reinforce the “normal” structure of water and the region of structure breaking disappears. In contrast, large less-hydrated ions disturb the ice-like structure and generate an extensive region of structure breaking. Structure-breaking ions are also referred to as chaotropes whereas structure makers are known as kosmotropes[11].In the author’s previous work[7], the flotation mechanism of kaolinite was found to be related to its unique face-edge structure. The presence of Na+ in the flotation pulp drastically interferes with the cationic flotation of kaolinite. In this work, the role of Na+, a water structure maker, and K+, a water structure breaker, in the cationic flotation of kaolinite, which has never been studied in the literature, was studied.
2. Experimental
2.1. Materials
- The kaolinite sample (Georgia, USA) was provided by Ward’s Natural Science Establishment. Quantitative X-ray diffraction analysis shows that the sample contains 93% kaolinite and 7% illite. The average particle size of the sample was determined to be 4.2 µm using a Malvern Mastersizer 2000. The sample was blended and riffled into 100 g charges for flotation tests. Flotigam EDA 3 (Clariant), an ether monoamine, and Flotigam 2835-2L (Clariant), an ether diamine, were used as collectors for kaolinite. Sodium chloride, hydrochloric acid and sodium hydroxide were ACS-certified chemicals from Ajax Finechem.
2.2. Methods
- The zeta potential of kaolinite particles was studied with the use of a Zetacompact Z8000 model (CAD Instrumentation, France). An electric field of 80 V/cm was applied on kaolinite suspensions. Results were based on an automated video analysis of particles. Zeta potentials were calculated from electrophoretic mobility using the Smoluchowski equation. The batch scale flotation tests were conducted in a modified 1 L laboratory stainless steel Denver cell in which the impeller was fitted with a variable speed drive and was driven from below, to allow the whole surface of the froth to be scraped with a paddle at a constant depth and rate[12]. For both conditioning and flotation the impeller speed was maintained at 900 rpm. Air was delivered to the cell at 4.8 L/min. Before flotation, 100 g kaolinite was added to the cell and first conditioned for 5 min while adjusting pH, and then conditioned with the added collectors for a further 2 min. Froth was scraped once every 5 sec. Distilled water was used in all the flotation tests.
3. Results and Discussion
- As Figure 1 demonstrates, the magnitude of the zeta potential of kaolinite particles over the entire studied pH range from 5 to 10 follows the Hofmeister series. The zeta potentials are more negative in the presence of sodium cations and less negative in the presence of potassium cations.
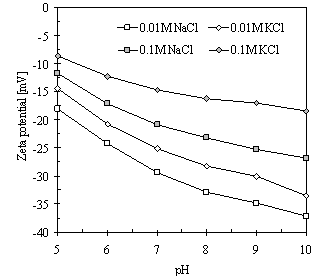 | Figure 1. Zeta potential of kaolinite in the presence of NaCl and KCl in the pH range from 5 to 10 |
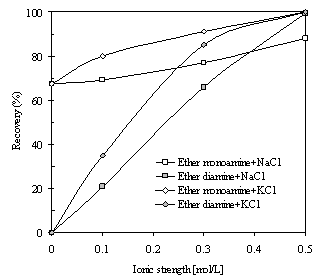 | Figure 2. The flotation recovery of kaolinite in the presence of NaCl and KCl, using 400 g/t ether monoamine and ether diamine as collectors (pH=10). |
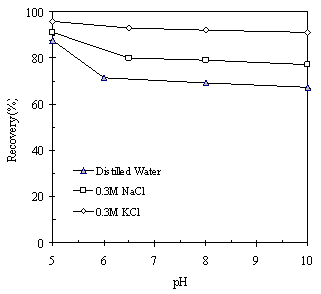 | Figure 3. Flotation recovery of kaolinite as a function of pH in distilled water, 0.3M NaCl and 0.3M KCl solutions, using 400 g/t ether monoamine as a collector |
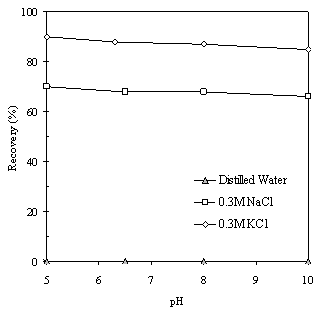 | Figure 4. Flotation recovery of kaolinite as a function of pH in distilled water, 0.3M NaCl and 0.3M KCl solutions, using 400 g/t ether diamine as a collector |
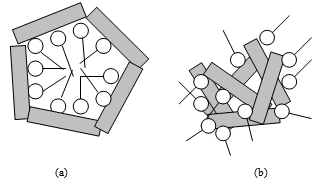 | Figure 5. Schematic description of the kaolinite-amines interactions. (a) Amine adsorption on dispersed kaolinite particles. (b) Amine adsorption on kaolinite particles coagulated by metal cations |
4. Conclusions
- The role of sodium and potassium cations in the flotation of kaolinite was studied using zeta potential measurements and batch scale flotation tests. It was found that both sodium and potassium cations increase the flotation recovery of kaolinite significantly, but the effect of potassium cations is stronger than that of sodium cations. As a water structure breaker, potassium cations can better adsorb onto the surfaces of kaolinite covered interfacial water structure, in comparison to sodium cations, and thus better reduce the zeta potential of kaolinite particles, which in turn causes stronger aggregation of kaolinite particles and higher flotation recovery of the clay mineral.
ACKNOWLEDGEMENTS
- The author would like to thank the Minerals Down Under, CSIRO National Research Flagship for support of this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML