-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Mining Engineering and Mineral Processing
p-ISSN: 2166-997X e-ISSN: 2166-9988
2012; 1(1): 1-16
doi:10.5923/j.mining.20120101.01
A Review on Novel Techniques for Chalcopyrite Ore Processing
Alafara A. Baba1, 2, Kuranga I. Ayinla3, Folahan A. Adekola1, Malay K. Ghosh2, Olushola S. Ayanda4, Rafiu B. Bale5, Abdul R. Sheik2, Sangita R. Pradhan2
1Chemistry Department, University of Ilorin, P. M. B. 1515, University of Ilorin, Ilorin, 240003, Nigeria
2Hydro & Electrometallurgy Department, Institute of Minerals and Materials Technology, Bhubaneswar, 751013, India
3Chemistry Department, Institute of Basic and Applied Sciences, P.M.B. 1375, Kwara State Polytechnics, Ilorin, Nigeria
4Department of Chemistry, Faculty of Applied Sciences, Cape Peninsula University of Technology, P. O. Box 652, Cape Town, South Africa
5Geology and Mineral Sciences Department, University of Ilorin, P.M.B. 1515, University of Ilorin, Ilorin, 240003, Nigeria
Correspondence to: Alafara A. Baba, Chemistry Department, University of Ilorin, P. M. B. 1515, University of Ilorin, Ilorin, 240003, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Chalcopyrite ores are usually processed by means of hydrometallurgical or pyrometallurgical processes, but due to environmental aspects and the possibility of increased exploitation of mixed and lower grade ores and relatively small isolated deposits, there has been a worldwide upsurge of interest in the hydrometallurgical processes of this ore as compared to pyrometallurgy. The different concentrates obtained through differential flotation in pyrometallurgy are usually of poor quality with low metal recovery. This further makes pyrometallurgical processing of chalcopyrite very difficult and costly and rendered them difficult to commercialize. As a result, the metal value is preferably extracted directly from low grade ores through hydrometallurgical process. A detailed review of chalcopyrite deposits, production and consumption, mining, ore processing, steps involved in the pyrometallurgical and hydrometallurgical processing of copper as well as the dissolution kinetics and microbial studies of chalcopyrite ore were discussed.
Keywords: Copper and Chalcopyrite, Ore processing, Hydrometallurgy, Pyrometallurgy, Dissolution Kinetics, Biohydrometallurgy
Cite this paper: Alafara A. Baba, Kuranga I. Ayinla, Folahan A. Adekola, Malay K. Ghosh, Olushola S. Ayanda, Rafiu B. Bale, Abdul R. Sheik, Sangita R. Pradhan, A Review on Novel Techniques for Chalcopyrite Ore Processing, International Journal of Mining Engineering and Mineral Processing , Vol. 1 No. 1, 2012, pp. 1-16. doi: 10.5923/j.mining.20120101.01.
Article Outline
1. Introduction
- Chalcopyrite is derived from the Greek words “chalkos”, copper and “pyrites” strike fire and it is also known as copper pyrite (Szymanowski, 1996). It is a brassy to golden yellow color mineral and was first discovered in Polk Country in 1847. The first mine opened in 1850 and first smelter was opened in 1885 and was operated until 1987 when it was closed due to unfavourable economics (Rotuska and Chimjeleniki, 2008). Chalcopyrite (CuFeS2) is the most common copper bearing mineral on earth (Nesse, 2000). Chalcopyrite is of primary importance and occurs in igneous and metamorphic rock and in metalliferous veins (McGraw-Hill, 1998). It contains many minerals including copper, zinc, sulfur and iron. All were produced at different times. At first, copper was the most important product and sulfur was a poisonous waste product. By the 1980's, 70% of production value came from the production of sulfuric acid (Hyvarinen et al. 2003). It is not only the most abundant of copper sulfides, but also the most stable minerals because of its structural configuration (face-centered tetragonal lattice). It is also the most refractory to hydrometallurgical processing (Haver and Wang, 1971). Chalcopyrite is the primary mineral which by alteration and successive enrich with copper produces the series, starting with chalcopyrite and going through bornites (Cu5FeS), covellite (CuS), chalcocite (Cu2S) and ending rarely as native copper (Brantley, 2003).At present, there are basically two main methods employed worldwide in order to process chalcopyrite for metal production. The most important one is the conventional - pyrometallurgy method: comprised numerous types of shaft and flash technologies, which consists of crushing, grinding, flotation, smelting, refining and electro-refining. This method is applied to sulphide flotation concentrates rather than ores and is economically feasible for copper rich feed for large scale operations. A second method is hydrometallurgy and this method is applied to the rest of the world’s primary copper production. Hydrometallurgy consists of crushing, leaching (non-oxidation leaching, atmospheric leaching and pressure leaching), solvent extraction and electrowinning. Hydrometallurgical processing can be effectively applied to oxidized ores containing CuO, Cu2O, carbonates and some silicates, as well as sulfide ores with chalcopyrite as a predominant copper mineral. Hydrometallurgical methods are used in countries having readily available deposits with low copper content with sulphur of oxidized form at the same time (USA, Chile, Australia and Peru) (Majima et al. 1985). Hydrometallurgical processing of chalcopyrite concentrates has received considerable attention over the last three decades (Haver and Wang, 1971).Approximately 70% of the world’s copper reserves are contained in the mineral chalcopyrite (Thomas, 2009). Thus, chalcopyrite, the most common ore of copper, occurs and widely distributed in metallic veins associated with pyrite (FeS2), phyrrhotite (Fe7S8), bornites (Cu5FeS), chalcocite (Cu2S), sphalerite (ZnS), galena (PbS), calcite (CaCO3), siderite (FeCO3), dolomite (CaCO3∙MgCO3) etc. Many carry gold or silver and became an ore of those metals. Often in subordinate amount with large bodies of pyrite (FeS2), making them serve as low-grade copper ores. Usually massive often making up golden sulfide mixture crystals over sphalerite surface (Szymanowski, 1996). Natural chalcopyrite has no solid solution series with any other sulfide minerals. There is limited substitution of Zn with Cu despite chalcopyrite having the same crystal structure as sphalerite (ZnS). However, it is often contaminated by a variety of other trace elements such as Co, Ni, Mn, Zn and Sn substituting for copper and Fe, Se and As substitute for sulfur, and trace amount of Ag, Au, Pt, Pd, V, Cr, In, Al and Sb are reported. It is most likely that many of these elements are present in finely inter-grown mineral within the chalcopyrite (Hershel, 2011). A large portion of the copper produced in the world is obtained by the smelting of chalcopyrite and ores associated with it (Yin et al. 1995).
1.1. Global Chalcopyrite Deposit
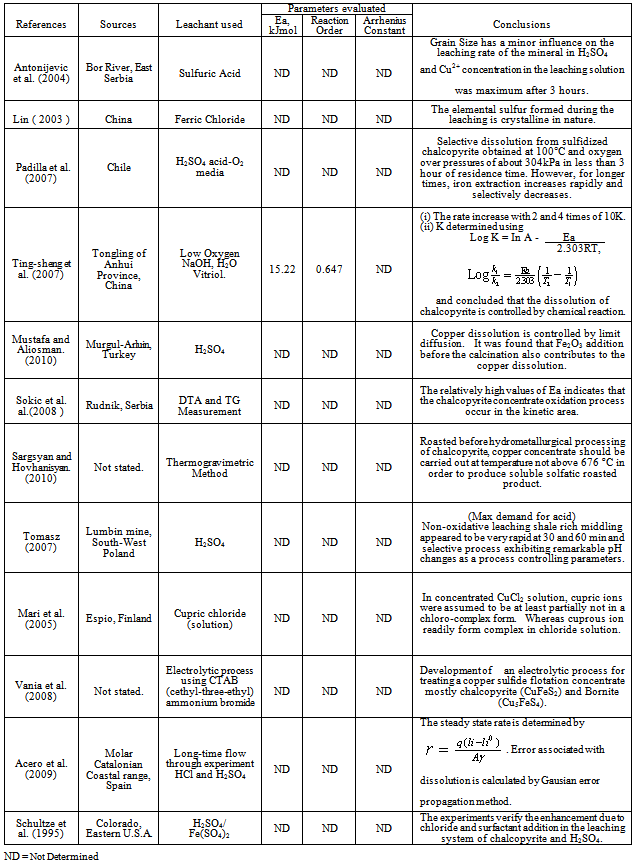
- Chalcopyrite deposits across the globe are as represented in Fig. 1. It is a fairly common mineral and therefore, only the finest of localities will be mentioned.Large, well shaped crystals occur in numerous places in Cornwell (England) as well as Akita, Ugo and Tochigi prefectures, Japan. Many fine crystals occur in the northern section of Mexico. Certain occurrences are La Bufa, Chihuahua, Charcas, San Luis Potosi and the Noche Buena mine, near Mazapil, Zacatecas Mexico (Hershel, 2011). The French Greek mine in Chester Co. Pennyslvania has produced huge crystal, many distorted and highly tarnished. Large amount of chalcopyrite occurs with sphalerite (ZnS), galena (PbS) and marcasite in the Joplin District of Missouri Okhlahoma and Kansas, USA.Chalcopyrite is widely distributed in the United States but usually in connection with other copper minerals in equal or greater amount found at Butte, Montana, Bingham, Utah, various districts in california, colorado, Arizona (Nevada, 2011). Many fine crystals occur in the northern section; certain occurrences are La Bufa, Chihuahua, Charcas - San Luis Potosi, and the Noche Buena mine near Mazapil, Zacatecas the sudburdy Canada. Chalcopyrite is also found in content metamorphic deposit in limestone (Bisbee, Arizona). Giant deposit crystals were found in Freirina, Northern Chile and also vast deposits are found in Northern Mexico (Hershel, 2011).Chalcopyrite is the copper ore deposit of Falum, Sweden of Namagaland in South Africa. However, vast crystals or deposits occur in Ishiagu, Ebonyi State; South Eastern region of Nigeria (West Africa). High great chalcopyrite deposit can also be found in Baluba, East of Lusaka (Zambia).A well shaped chalcopyrite crystal occurs in many places such as Akita, Ugo and Tochigi prefectures Japan (Chuck and Virginia, 2011).Large, well shaped crystals of chalcopyrite also occur in numerous places in Falum (Sweden), Rio Tinto (Spain), Mansfeld (Germany), Lukkulaisvaara Ultrabasic Massif, Olanga (Oulanka) River, Ounlanka Plutonic complex and Karelia Republic (Northern Region, Russia).
 | Figure 1. Chalcopyrite deposit across the Globe (Facts about copper, 2010) |
1.2. Chalcopyrite Ore Production
- The sulfide ore minerals including chalcopyrite (CuFeS2), sphalerite (ZnS), galena (PbS) provide the major sources of the world’s base metals (Cu, Zn, Pb, respectively), whereas pyrite is virtually ubiquitous as a metalliferous mineral in sulfide ore deposits (Crang and Vanghan, 1994). The first step in recovering metal from their mineral is that of finding ore-bodies in which metal sulfides are present in a sufficient quantity and as a result of oxidation of the sulfide mineral in nature weathering underground. The induced polarization exploration technique involves applying a series of current pulses to two electrodes inserted into ground and analyzing the induced voltage at two different electrodes also inserted into the ground (Jones, 1999). Valuable minerals are thus extracted from the ore by means of flotation separation, extractive metallurgy or leaching.
 | Figure 2. World Trend in (SX-solvent extraction) capacities from 1985 – 2005 (Rotuska and Chimjeleniki, 2008) |
1.2.1. Flotation Separation
- Valuable mineral in an ore such as chalcopyrite ore can be separated from each other and from worthless gauge minerals by the froth flotation process. The process was developed in Australia at the start of the 20th Century to treat the primary sulfidic silver/lead/zinc ore at Broken Hill (Woods and Doyle, 2000). Many approaches were pursued to solve the sulfide problem at Broken Hill before selective flotation was developed. The successful technology involve first crushing the ore, typically to a particle size of about 5 to 50 micrometer to liberate separate grains of the various valuable mineral and worthless gauge components. Then the particle is pulped with water and the surface of the mineral of the interest selectively made hydrophobic through the addition of organic specie which is term collector. Following this procedure, a stream of air bubbles is passed through the pulp; the bubbles attach to levitate the hydrophobic particles and are collected in a froth layer that disengages from the flotation cell by flowing over the weir of the cell. A frother such as a long chain alkyl alcohol is added to create a stable froth layer in the cell. The collector used at Broken Hill in the early days was eucalyptus oil derived from the leaves of the ubiquitous Australia “gum” tree. Following the success at Broken Hill, mining companies throughout the world rapidly adopted the flotation process (Young et al. 2003). Flotation also used to split copper and nickel sulfides and separate them from iron sulfides and gangue minerals. Cobalt appears in the nickel concentration and the cobalt is subsequently recovered. Flotation has developed from the treatment of simple ores to the ever more complex ones that are being found today. Thus, for example, the three metals in copper/lead/zinc ore are floated into three concentrates, each containing one of the metals (Pletcher et al. 1993).
1.2.2. Extractive Metallurgy
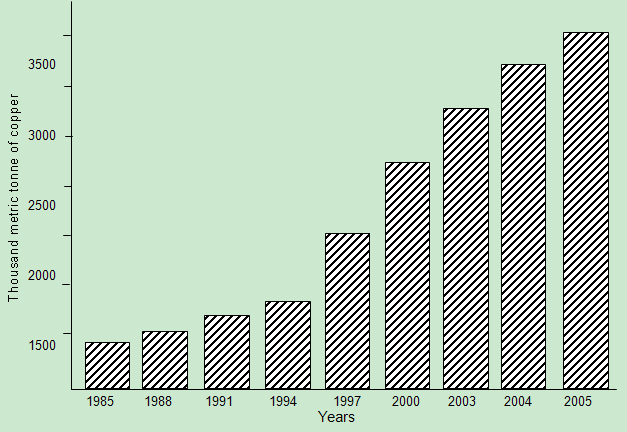
- Metal sulfide flotation concentrates occur in a number of ways. These include using pyrometallurgy or smelting at high temperature. In this process, the sulfur in the metal sulfide is oxidized with air or oxygen to sulfur dioxide and molten matte is produced.Flotation concentrates can also be processed using hydrometallurgy in which the metal sulfide is dissolved (leached) into an aqueous solution (Bergy and Yianatos, 2001). Most oxide of copper minerals dissolves readily in sulfuric acid so that leaching is reasonably straight forward. A concentrated, pure copper sulfate solution suitable for electrowinning is produced from the initial leach solution by selectively transferring the copper ion to an organic phase by a process known as solvent extraction and returning them again to an aqueous phase (Young et al. 2003). Complexing agents are dissolved in an organic solvent at low vapour pressure kerosene and this phase is intimately dispersed within the aqueous leach solution in a mixer-settler. The copper selectively complexes with the reagent and is transferred into the organic phase. The copper replace hydrogen ion in the complex and hydrogen ions are subsequently transferred to the aqueous phase. The two phases are allowed to separate in the settler section and then the aqueous acid solution termed the “raffinate” is recycled to ore leaching. The loaded organic phase is sent to a second mixer-settler where it is reacted with a strong aqueous sulfuric acid solution. Here, the copper is exchanged for hydrogen ions and transferred into the second aqueous phase from which the metal is efficiently recovered by electrolysis. The overall process of dissolution, solvent extraction and electrowinning is known as SX-EW. Fig. 3 shows a typical flow diagram of LX/SX/EW.
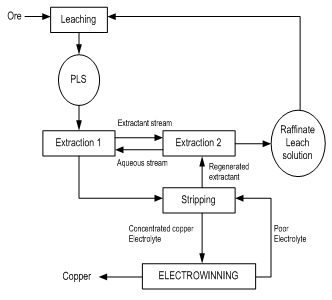 | Figure 3. Typical flow diagram of LX/SX/EW (Bergy and Yianatos, 2001) |
1.2.3. Leaching
- Leaching is a heterogeneous reaction that takes place at the interface between a solid and liquid phase and sometimes a gaseous phase. At the boundary between the two phases, a diffusion layer is formed. The dissolution of mineral ore takes place through the following stages: (1) diffusion of reactant through the diffusion layer, (2) adsorption of the reactant on the solid, (3) chemical reaction between the reactant and the solid, (4) desorption of the product from the solid and (5) diffusion of the product through the diffusion layer. Any of these stages (1) - (5) may be the rate controlling step depending on its relative speed to the others as represented in Fig. 4.
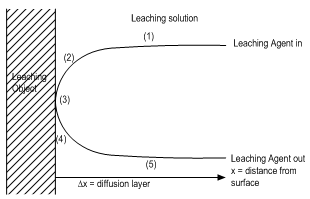 | Figure 4. Basic sketch of a leaching process (Crest, 2000) |
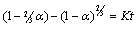 | (1) |
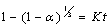 | (2) |
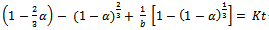 | (3) |
1.3. Chalcopyrite Ore Consumption
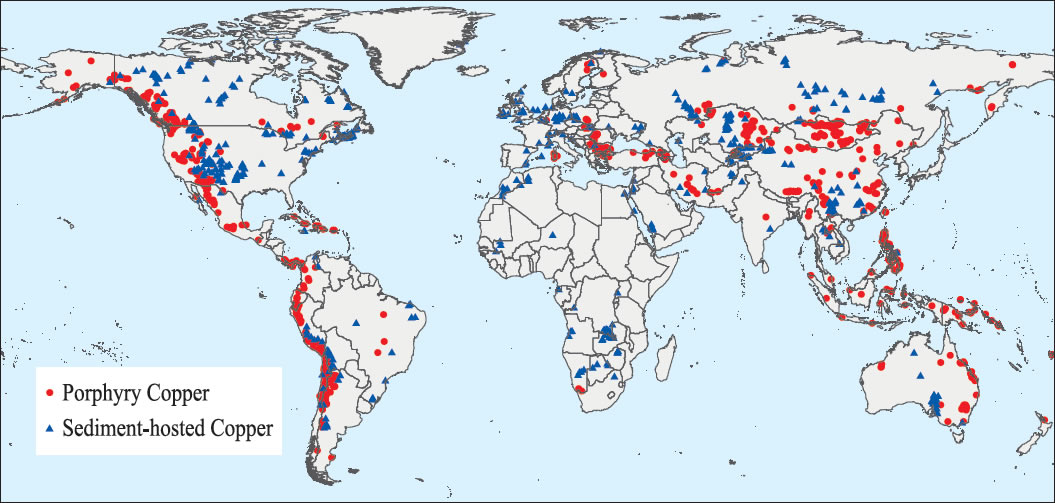
- Approximately 70% of the world’s copper reserves are contained in the mineral chalcopyrite. Currently, copper is extracted from high-grade chalcopyrite through smelting a high temperature process with undesirable environmental side effects (Thomas, 2009)The world’s production (supply) and consumption (demand) of chalcopyrite (copper) have increased dramatically in the past 25 years. As large developing countries have entered the global market, demand for mineral commodities has increased. In the last 20 years, the Andean region of South America has emerged as the world most productive copper region. In 2007, about 45% of the world’s copper was produced from the Andes Mountain (USA). The United State produced 8%, virtually all copper produced in the United State comes from Arizona, Utah, Mexico, Nevada or Montana in decreasing order of production.The risk of distribution to the global copper supply is considered to be low because copper production is globally dispersed and is not limited to a single country or region due to its importance in construction and power transmission. However, the impact of any copper supply distribution would be high. The qualities of copper that have made it the material of choice for a variety of domestic, industrial and high-technology applications have resulted in a steady rise in global copper consumption. US geological survey studies of copper consumption showed some interesting trends for the 1980 to 2008 time period. Copper consumption in emerging economies such as China and India rose considerably, whereas the consumption rate in industrialized economies such as the United States fell slightly until 2002. The United States was the leading copper consumer and artificially use about 16 percent of total world refined copper (about 2.4 million tonnes). In 2002, the United States was overtaken by China as the world’s leading user of refined copper. The booming economy in China contributed to a tripling of its annual refined copper consumption during the 8 years from 1999 to 2007. The global consumption of copper from 1980 to 2008 is shown in Fig. 5.Copper is one of the most widely recycled of all metals and approximately one-third of all copper consumed worldwide is recycled. Recycle copper and its alloy can be re-melted and used directly or further reprocessed to refined copper without losing any of the metals’ chemical and physical properties (Dixon and Dreisinger, 2003).
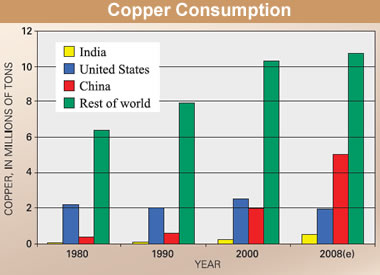 | Figure 5. Global consumption of copper from 1980 – 2008 (USGS, 2009) |
1.4. Market Potentials and Industrial Applications of Copper
- Copper was one of the first metals ever extracted and used by humans and it has made vital contributions to sustaining and improving society since the dawn of civilization. As man learned to fashion his weapon from iron and steel, copper began to assume another roles. Being a durable metal and possession of great beauty, it is used extensively for household utensils, water pipe, marine uses and other purposes that required resistance to corrosion. Copper is easily stretched, molded and shaped. It is resistance to corrosion and conducts heat and electricity efficiently. The unusual ability of this mineral to conduct electric current account for its greatest use today (McGraw-Hill, 1998). As a result, copper was important to early human and continue to be a material of choice for a variety of domestic, industrial and high-technology applications today (USGS, 2009).Copper producers all around the world are facing continuously increase demands, both economically and environmentally. During the last decade, the production chain of copper from chalcopyrite has shifted more and more into direction where copper is smelted by custom smelters far away from copper mines (Jukka and Likka, 2005).The first commercial plant for chalcopyrite treatment started at the Rancher’s Bagdad Mine in 1968 with a production capacity of 6,000 tons per annum. The first large plants was bought into operation in Zambia to produce 100,000 tons per annum in 1973 and by 1980 new plants were on stream in the USA, Mexico and Chile to produce a total production capacity of approximately 180,000 tons per annum. Today, there is a total world market potential of 20.4 million tons which costs at 110 US dollar per ton (Seward, 2001).
2. Chalcopyrite Ore Extraction Techniques
2.1. Mining of Chalcopyrite
- Chalcopyrite is a common mineral and is found in almost all sulfide deposit and is often disseminated through igneous rock (Haver and Wang, 1971). For lower grade deposit located near the surface, the open - pit method is the most practical for the mining of large tonnages of material. Large track mounted drill of ore is prepared for blasting and the broken ore is hauled to the ore dressing plant by truck (at up to 150 tons per load) or conveyor. In underground mining, vertical shaft are sunk well over 1,000 metre (3,300 ft) below the surface and channels are extended to the ore body. The ore, broken by drilling and blasting is hoisted through the shaft and conveyed to the processing plant. In some cases, primary crushing takes place underground and in others, a ramp and trucks carry ore to the surface (McGraw-Hill, 1998).
2.1.1. Factors Affecting the Mining of Chalcopyrite
- Factors which may contribute to the decision to mine or not to mine chalcopyrite mineral resources include the following:(a) The availability of the rock or ore is obviously necessary: When mining begins, the richest sources of ore are mined first and lower grade ore is mined later. So, as time passes, the cost of mining tend to goes up and this was the case with iron mining and phosphate in Tennessee.(b) The availability of alternative sources of materials also affects decision to mine: The discovery of vast amount of bird guano in South America in the late 19th Century destroyed the Saltpeter industry in Tennessee.(c) Change of technology affects mining decisions: The making of tools from chart stopped when iron tools were introduced. The quarrying of dimension limestone practically stopped when techniques of making and using cement improved.(d) Governmental policy also affects decision of mining: Saltpeter mining flourished Tennessee in early 19th Century as a result of large-scale US government purchase of saltpeter to make gunpowder for the war of 1812.(e) Pollution either in the mining or from the use of the finished product can affect mining decisions, either by increasing the cost of production or by increasing the demand for the product (Rotuska and Chimjeleniki, 2008).
2.2. Chalcopyrite Ore Processing
- The first step in the chalcopyrite processing is to liberate the copper minerals and remove waste constituents such as alumina, limestone, pyrite and silica so that the copper and non-ferrous mineral values are concentrated into a product containing between 20 to 30 percent copper. The second step involves either smelting or leaching to remove a large proportion of impurity particularly iron and in the case of sulfides ore, sulphur. The final step, refining, removes the last traces of the impurity elements and produces a copper product of 99.99 percent purity (McGraw-Hill Encyclopedia, 1998).Once the chalcopyrite concentrate containing copper and other metals of value (such as gold, silver) has been produced, the next step is to remove impurity element. In older processes, the concentrate containing between 56 - 10 percent water, is first roasted in a cylindrical, refractory lined furnace of either the hearth or fluidized bed type. As concentrate is fed into the roaster, it is heated by a stream of hot air to about 590℃. Volatile impurities such as arsenic, mercury and some of the sulphur are driven off, the sulphur being removed as sulphur dioxide (SO2). What remains is an oxidized product containing a percentage of sulphur that is sufficiently low for smelting. This is traditionally done in a reverberatory or electric furnace into which concentrate is fed along with a suitable amount of flux. These are heated by combusted fuel or electrical current to a temperature of 1230℃ – 1300℃, producing an artificial copper iron sulfide that settles in a molten pool at the bottom of the furnace (McGraw-Hill, 1998).In the roaster, the copper concentrate (chalcopyrite) is partially oxidized to produce alkaline (Cu2S) and sulphur dioxide (SO2) gas. The chalcopyrite ore is heated strongly with silicon - dioxide (silica) and air or oxygen in the furnace or series of furnaces. The copper (II) ion in the chalcopyrite is reduced to copper (I) sulfide which is reduced further to copper metal in the final stages. The iron in the chalcopyrite ends up converted into an iron (II) silicate slag which is removed. Most of the sulfur in the chalcopyrite turns to sulfur dioxide gas. This is used to make sulfuric acid via the contact process (copper extraction and purification). Overall equations for these series of steps are:
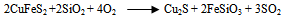 | (4) |
 | (5) |
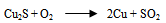 | (6) |
2.2.1. Smelting Process (Pyrometallurgy)
- Pyrometallurgical practice typically involves smelting converting, anode casting and electro-refining of the anodes to high purity copper metal. The smelting and refining processes used well established technologies are energy efficient and have high metal recoveries including those of gold and silver (Yin et al. 1995). This begin with a dry concentrate containing less than one percent water, which along with flux is contacted in a furnace by a blast of oxygen or oxygen enriched air (McGraw-Hill Encyclopedia, 1998). Pyrometallurgical extraction involves heating the mineral cake in a blast furnace; oxygen pressure and temperature are carefully controlled. The first stage involve the separation of copper and iron ore (Equation 7), followed by the addition of silica (SiO2) to the blast furnace to convert iron (II) oxide to a less dense liquid layer of slag, iron (III) silicate which is poured off.
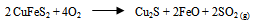 | (7) |
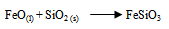 | (8) |
 | (9) |
 | (10) |
 | (11) |
2.2.2. Leaching Process (Hydrometallurgy)
- Hydrometallurgy is applied to the rest of the world’s primary copper production, objected mainly on oxide and/or low grade copper ore due to significantly lower operating cost (Dresher, 2001). Treatment chain in hydrometallurgy processes are usually consisted of crushing, leaching (non-oxidative leaching, solvent extraction and electrowinning). Hydrometallurgical processing can be effectively applied to oxidized ores containing CuO, Cu2O, carbonate and some silicates and rarely for sulphide ore with chalcocite and covellite as predominant copper mineral (King, 2007; Stevanovic et al. 2009).Beginning of solvent extraction process development objected on metal extraction from leach solutions, date from the 60s of last century. Beforehand, solvent extraction processes were used in analytical chemical assaying (Habashi, 2007). Hydrometallurgical methods are used in countries having readily available deposit with low copper content and with surplus of oxidized forms at the same time (USA, Chile, Australia and Peru). The most important development in copper hydrometallurgy with respect to the growing number of its application as well as for its future potential has been solvent extraction process. It becomes the achievement which revolutionized copper production all over the world and enabled to introduce hydrometallurgy for industrial scale (Stevanovic et al. 2009). Occasionally, it is adopted in preference to smelting or pyrometallurgy and is carried out at lower temperatures and thus eliminates the generation of sulfur dioxide. There are, however, effluents and residues that must be treated in order to protect environment. In the hydrometallurgical processes, the ore or concentrate (chalcopyrite) is brought into close contact with a leach solution (frequently sulfuric acid) that dissolves the copper and leaves a residue of gangue (and frequently precious metals). Various system, some quite complex are used to bring copper minerals into contact with the leach solution, wash and filter the residue and finally purify the solution to remove dissolved iron and other impurities (McGraw-Hill, 1998). Some experimental hydrometallurgical techniques to process chalcopyrite are being investigated, but as of 2009 are unproven outside the laboratories. Some rod leach or pressure leach process exist to solubilise chalcocite concentrate and produce copper cathode from the resulting leach solution, but this is a minor part of the market.
2.2.2.1. Chloride Leaching
- Only chloride of metal in a high valence state such as ferric or cupric will leach metal from their sulfides because oxidation is necessary. Of the numerous chloride routes, ferric chloride (FeCl3) leaching of chalcopyrite concentrates which was initiated by the U.S. Bureau of Mines, Reno Metallurgy Research Center in 1969 received significant attention (Philips, 1976).
2.2.2.1.1. Intec Copper Process
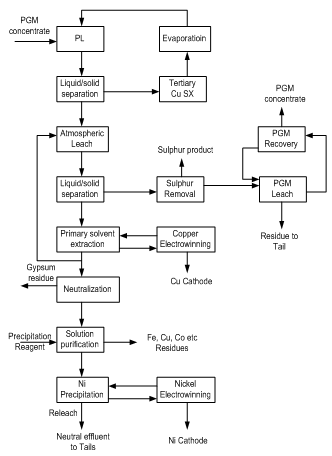
- The intec copper process is a proven, patented hydrometallurgical process for the extraction of pure copper and precious metal from sulphide concentrates. The process is based on the electrolytic deposition at the cathode of LME (London Metal Exchange). Grade A purity copper from a purified sodium chloride, sodium bromide electrolyte during electrowinning; the mixed halide species BrCl2 is generated, characterized when it is re-circulated to treat incoming concentrate feed (Crest, 2000).Description of Intec Copper ProcessThe patented intec copper process was developed for the recovery at LME Grade A purity from sulfide concentrates. Essentially, the intec copper process consists of the three sequential circuits: leaching, purification and electrowinning. A simple flow diagram for the intec copper process is shown in Fig. 6.
2.2.2.1.2. The Cuprex Process
- The cuprex process leaches chalcopyrite concentrate at atmospheric pressure with ferric chloride solution in two stages. The pregnant liquor containing copper, iron and minor amount of impurities mainly zinc, lead and silver is sent to the extraction stage of the SX circuit where it is contacted at ambient temperature with a kerosene solution DS5443. The copper, selectively transferred to the organic phase is extracted in three stages. The aqueous solution of copper chloride is then sent to the electrolysis section as catholyte, which is fed to the cathode compartment of an electrowinning cell to produce granular copper. Electrowinning of copper from chloride solution take place in a diaphragm cell where the cathode and anode compartment are separated by a reinforced cation-selective ion-exchange membrane. Chlorides generated at the anode is recovered and used to deoxidize the cuprous chloride generated in the catholyte during electrowinning.
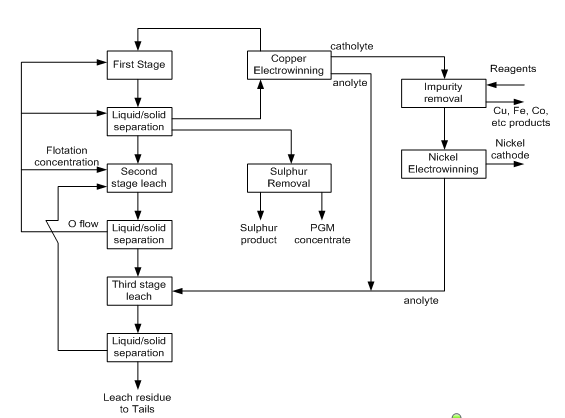 | Figure 6. Conceptual block diagram for PGM and base metal recovery by the intec process (Crest, 2000) |
2.2.2.2. Pressure Sulfate Leaching
- Thermodynamically favorable at an elevated pressure, the use of high pressure in chalcopyrite concentrate leaching has led to a reliable and cost competitive process option. A strong resurgence of interest in the pressure sulfate leaching of chalcopyrite concentrates occurred after developments in construction of materials, more efficient mills for fine or ultrafine grinding of sulfides and successful implementation of autoclave technology for zinc and refractory metals (Jones, 1999). Based on the literature data for sulfide leaching especially chalcopyrite in an oxidizing acidic medium and the characterization of the leach liquor and residues obtained, the following chemical reactions were chosen to study CuFeS2, H2SO4, NaNO3 and H2O systems. The main reactions include:
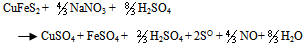 | (12) |
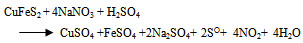 | (13) |
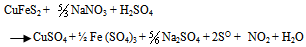 | (14) |
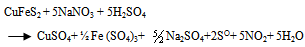 | (15) |
2.2.2.2.1. The CESL Process
- The CESL process is a divergent approach to low pressure oxidation in which a high proportion of sulfide (sulphur) remain in the elemental form in leach residue (McCunn et al. 2004). This process also employ a chloride enhanced oxidative pressure leach in a controlled amount of acid designed to convert the copper to a basic copper sulfate salt, the iron to haematite and sulfur to elemental sulfur. The flow diagram of a CESL process is shown in Fig. 7.
2.2.2.2.2. Dynatec Process
- The dynatec process involves oxidative leaching of chalcopyrite concentrate at 150oC using coal at a modest dosage (25 kg/t of concentrate) as an effective anti-agglomerant (Collins and Kofluk, 1998). Sulfide oxidation chemistry is similar to the CESL process. A high extraction of copper (98%) is achieved by either recycling the unleached sulfide to the leach after the floating and removal of elemental sulfur (melting and filtration) or pre-treating the concentrate with a fine grinding (+90 - 25µm).
2.2.2.2.3. Total Pressure Oxidation
- The total pressure oxidation uses water as the leach medium and converts most of the sulfide sulfur to copper sulfate and sulfuric acid (King et al. 1994). Reground concentrates is slurried in acidic recycle solution and pumped into the first compartment of an autoclave operated at 210 – 220 oC, with sparked oxygen to maintain 700 kPa of over pressure. Basic iron sulfate (hydroniumjarosite) is a significant co-product which will collect silver and other monovalent cation.
 | Figure 7. Schematic drawing of the column leach apparatus (Dresher, 2004) |
2.2.2.2.4. Brisa Process
- Brisa process is based on bioleaching by indirect mechanism which involves two separate biological and chemical stages. In the chemical stage, chalcopyrite concentrate are leached with ferric sulfate at 12 g/L and pH 1.25 in the agitated reactors using silver as a catalyst. Higher copper extraction (>95 % wt) is obtained by activating concentrates with 2 mg Ag/g of concentrate at 70 oC and 10 hours leaching (Dresher, 2004).
2.2.2.2.5. The Biocop (TM) Process
- BHP Billiton and Codelco built a demonstration plant at Chuquicamata in North Chile (Dresher, 2004). This was constructed in a stirred reactor containing dilute sulfuric acid into which air is blown, hydrothermophilic microorganism which operate at a temperature between 60oC and 90oC are used. Leaching of chalcopyrite concentrate is complete within 10 days (Dresher, 2004).
2.2.2.2.6. The Bac Tech/Minitek Process
- Bac Tech and Minitek working in conjunction with industries Penoles SA de CV, operated a 2.2 c/d demonstration plant for the bioleaching of copper concentrate in Monterrey, Mexico (Miller, 1999). Conducted in a series of counter current reactors, the thermophilic microorganisms are used at temperature of 25 – 55℃, pH 0.5 - 2.5 is maintained within the reactors. Carbon dioxide is obtained from ambient air, nutrients are added to the leach liquor and retention time is about 30 days. The plant employs moderate thermophilic to oxidize the sulfides followed by conventional SX-EW to recover the contained copper, which achieved recovery rate of 96.4 % and a residency period of six days (Dresher, 2004)
3. Dissolution Kinetic Studies on Chalcopyrite Ores
- Various reported works on the dissolution kinetics and solvent extraction of copper from chalcopyrite ores are discussed thus. It is important to note that limited data in this area of research from Nigeria origin has been documented (Olubambi et al. 2006). These authors did not address some kinetic parameters such as activation energy (Ea), reaction order, Arrhenius constant etc. for the better prediction of the dissolution mechanism that could be used in the selective and purification of copper from the ores by solvent extraction technique. A study reported by Lu (1982) studied the effect of chloride ions on the dissolution of the mineral in oxygenated acid solution and they concluded that the presence of sodium chloride (NaCl) in the leaching solution promoted the formation of porous sulphur layer, favouring the diffusion of the leaching reagents through the film product of the reaction continuation in the surface of the mineral. Winand (1991) also studied the addition of sodium chloride (NaCl) in the copper sulphide leaching that attributed to the formation of copper chloride complex ion as being a decisive factor for increasing the leaching rate for increasing the copper solubility in the system Cl-/Cu (II) and/ or Cu (I). He suggested that NaCl act on the elemental sulphur layer and also on the solubility of copper complexes in the reaction system with aim to improving the copper extraction from the sulfide mineral in a relatively short time with low operation cost, taking into consideration the influence of a cationic surfactant. Vania-Mori et al. (2009) studied the advantages of copper sulphide CTAB (Cetyl-three-ethyl ammonium bromide) on the electro-leaching of copper sulfide (chalcopyrite) flotation concentrate. The result of the electro-leaching process shared that extraction of copper in the presence of surfactant cation CTAB had an increased extraction compared to the results of the one without CTAB. The test shows evidence that the electro-leaching process makes possible the production of chlorate ion that dissolves the copper sulphides releasing copper ion to solution, which are simultaneously reduced in the cathode surface.Olubambi et al. (2006) studied the leaching of zinc and copper from Nigeria bulk sulfide ore with sulfuric acid in the presence of hydrogen peroxide (H2O2), with the aim to investigate the effectiveness of hydrogen peroxide as an oxidant for the sulfuric acid. The results of this study have shown that sulfuric acid leaching in the presence of hydrogen peroxide is an effective route for copper and zinc recovery from Nigerian complex sulfide ore.The dissolution process in the presence of H2O2 has been investigated by both Jiang et al. (2002) and Adebayo et al. (2003). The dissolution take place in two reaction stages; viz: the dissolution of elemental sulphur and its conversion to sulfate (SO42-) which can be expressed as follows:
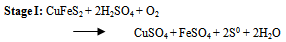 | (16) |
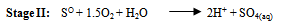 | (17) |
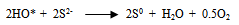 | (18) |
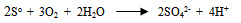 | (19) |
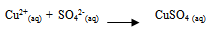 | (20) |
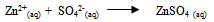 | (21) |
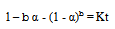 | (22) |
3.1. Microbial Leaching Studies on Chalcopyrite
- Biohydrometallurgy treatments have been practiced extensively for metal recovery from low-grade sulfide - bearing materials (Maouf, 1971). The thiobacillus ferrooxidans which is responsible for metal dissolution derives the necessary energy for its life process from oxidation of ferrous iron (Silverman, 1967) and reduced valence inorganic sulfur compounds (Silver and Torma, 1974) and thus utilizes carbon dioxide for growth when cultured on metal plates.Most studies to date, Biswas and Davenport (1980) and Torma et al. (1972) have been concentrated on improving both the kinetic of copper dissolution by using a large number of bioleaching species (mesophilic and moderately or extremely thermophilic microorganisms, and the knowledge of how certain physical factor (particle size, temperature, pH, catalyzing ions) affect the process. The results thus far obtained have made possible to establish the most suitable conditions for carrying out the bioleaching process, but have not revealed the cause which bring about a decrease in the extraction rate after certain time.Gomez et al. (1995) used culture which is principally formed for Thiobacillus ferroxidans, Thiobacillus thioxidans and leptosipinillum ferooxidans. The result of their investigation showed that the reactivity of the chalcopyrite gradually increase with the bacterial treatment. Karanam et al. (2007) carried out bioleaching studies on periolatin columns using low-grade copper containing rock (granite). The experiment made use of lixiviant consisted of acidified ferric sulfate containing acidophilic microorganism. Leaching parameters studied were lixiviants flow rate, particle size, and bed height. The results showed that the leaching efficiency increases with decrease in particle size and lixiviant flow rate.According to Nowaczyk et al. (1998), the Thiobacillus ferrooxidans bacterial for processing copper and iron leaching from chalcopyrite from (Kotlina Ktodska) was investigated. Their results showed that the leaching of metals from chalcopyrite occurs according to the following reactions:
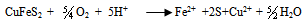 | (23) |
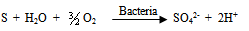 | (24) |
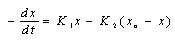 | (25) |
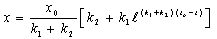 | (26) |
|
4. Conclusions
- Hydrometallurgical treatment of chalcopyrite (CuFeS2) is a growing technology (Hyvarinen and Hamalainen 1999). In recent times, attention has been drawn to low-grade complex sulfide ores due to the decline in the world deposits of high - grade ores. Complex sulfide ores are in many cases difficult to treat with conventional mineral processing method and the concentrate produced is often not clean enough. This seriously limits their commercial value (Fan et al. 2005). Differential flotation does not release all the constituent phases and so the different concentrates obtained are of poor quality with low metal recovery. This makes further pyrometallurgical processing of these ores very difficult and costly (Hyvarinen et al. 2003) and rendered them difficult to commercialize. Consequently, the metal value is preferably extracted directly from the low grade ores through hydrometallurgical process (Hiroyoshi et al. 2001). The basis of hydrometallurgy is justified not only by economical, but also ecological reasons. Increasing demand of industry for metals leads to a quick exhaustion of the best or the easiest accessible resources which stimulate the search for new environmental - friendly solutions such as bio-hydrometallurgy to enable exploitation of poor deposit or recovery of metal from industrial wastes. In general, experiences have been shared based on various researches on hydrometallurgical process and now we find that many deposit around the world that have previously not been regarded as suitable for economic exploitation are being re-considered and this will hopefully lead to the valuable economic regeneration of other mineralogical areas.In conclusion, it is pertinent to note that there are two main methods employed worldwide for processing chalcopyrite for metal production. The most important one is the conventional - pyrometallurgy method comprised numerous types of shaft and flash technologies, which consists of crushing, grinding, flotation, smelting refining and electro-refining. This method is applied to sulfide flotation concentrates rather than ores and is economically feasible for copper rich feed for large scale operations. A second method, hydrometallurgy is applied to the rest of the world’s primary copper production. Hydrometallurgy consists of crushing, leaching (non-oxidation leaching, atmospheric leaching and pressure leaching), solvent extraction and electrowinning and can be effectively applied for oxidized ores as well as for sulfide ores with chalcopyrite as a predominant copper mineral. In general, due to interests that include environmental aspects and the possibility of increased exploitation of mixed and lower grade ores and relatively small isolated deposits, there has been a worldwide upsurge of interest in hydrometallurgical process for the production of copper.
ACKNOWLEDGEMENTS
- A. A. Baba wishes to thank University of Ilorin for permission to honour CSIR-TWAS Fellowship award; Prof. B. K. Mishra, Director, Institute of Minerals and Materials Technology, Bhubaneswar-751013, India for the acceptance as CSIR-TWAS Postdoctoral Fellow; the Management, Academy of Sciences for the Developing World, Trieste, Italy and Council of Scientific and Industrial Research, New-Dehli for the Award of 2010 CSIR-TWAS Fellowship for Postdoctoral Research (May, 2011-January, 2012).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML